Mastering pH and pOH: The Critical Answers Solving Problem Set 9
Mastering pH and pOH: The Critical Answers Solving Problem Set 9
In the intricate dance of aqueous chemistry, pH and pOH stand as fundamental pillars defining the acidity or alkalinity of solutions—a balance so vital it governs everything from environmental health to medical treatments. Problem Set 9 challenges learners to decode the relationships between hydrogen ion concentration, hydroxide ions, and the logarithmic functions that simplify these complex interactions. Understanding the answers to this set requires more than rote memorization; it demands a clear grasp of equilibrium, logarithmic principles, and real-world applications, making the mastery of pH and pOH not just academic, but profoundly practical.
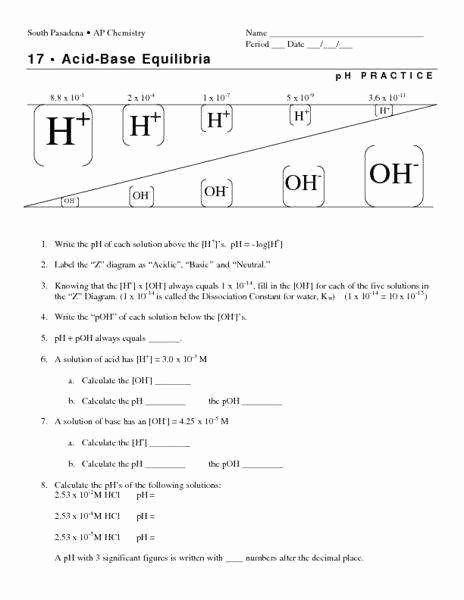
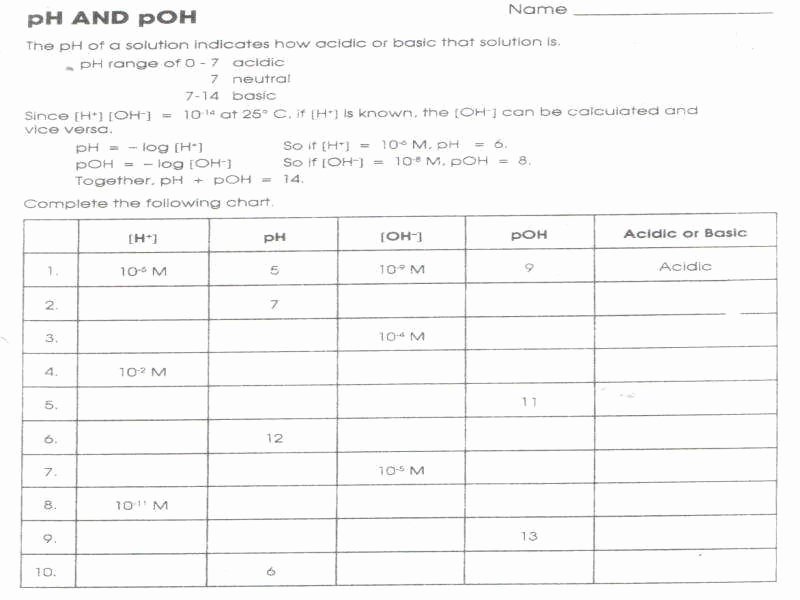
At the heart of the problem lies the quintessential equation: pH plus pOH equals 14 at 25°C, a constant derived from the ion product of water—Kw = 1×10−14. This relationship forms the backbone of problem-solving, revealing how increasing one ion’s concentration necessitates a compensatory shift in the other. When a solution’s pH is measured, pOH becomes instantaneous: pOH = 14 – pH.
Reverse logic follows—knowing pOH allows precise calculation of [OH−], and from there, critical insights into reactivity and stability.
The Chemistry Behind the Numbers
The pH scale measures hydrogen ion activity on a base-10 logarithmic foundation: pH = −log10[H+]. Similarly, pOH uses hydroxide ions: pOH = −log10[OH−]. These scales compress wide concentration ranges into digestible numerical values: pH 0 through 14 covers nearly pure acids to strong bases.The inverse logarithm ensures even minute changes in ion concentration translate to distinct pH values—making pH a powerful tool for monitoring chemical systems. Crucially, pH operates only in aqueous environments, where water dissociation dictates this equilibrium. “pH is not simply a number—it’s a concise, dynamic descriptor of chemical potential,” notes Dr.
Elena Torres, environmental chemist at the National Standards Laboratory. “Each digit reflects a tenfold shift in ion activity: a difference of one pH unit means ten times more acid, ten times fewer hydroxide ions.”
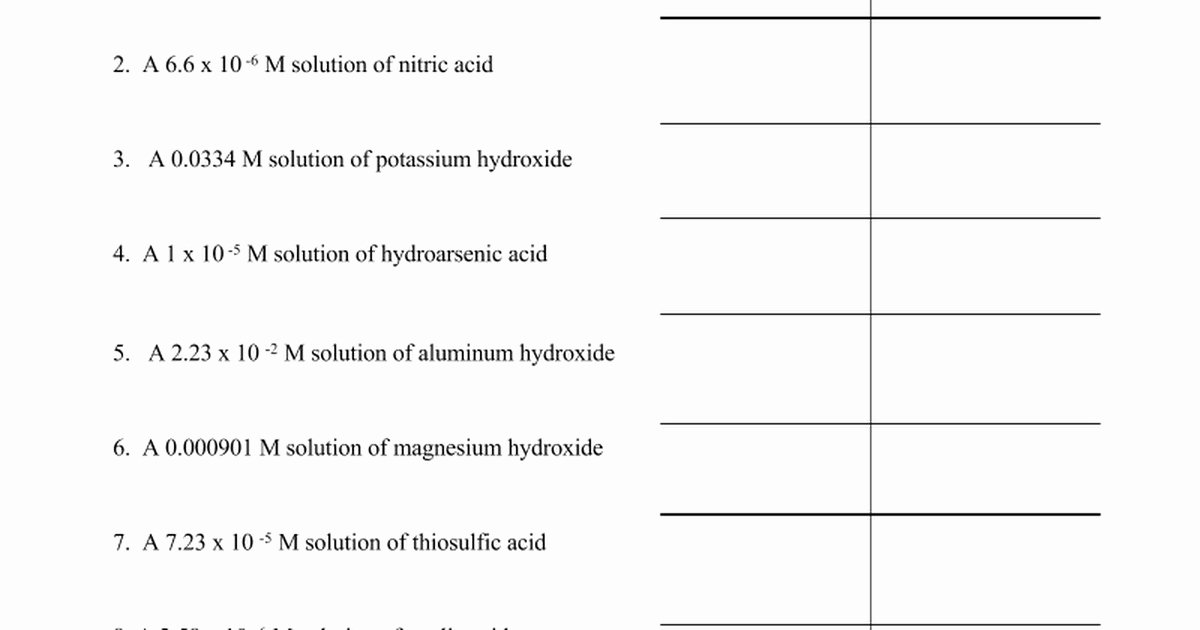
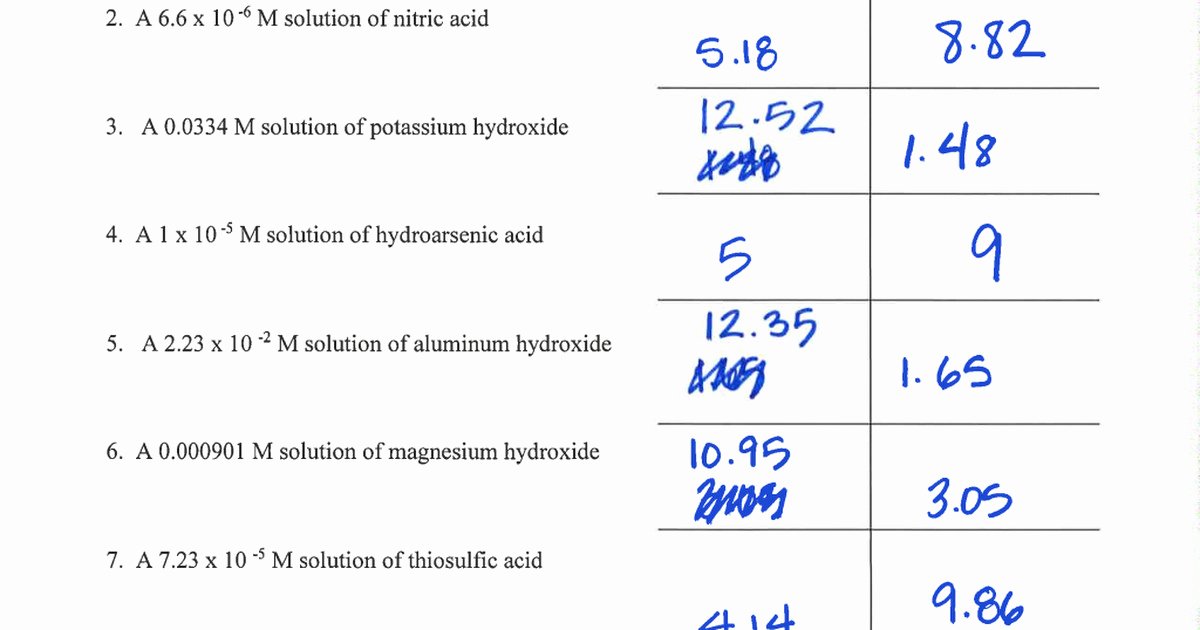
Problem Set 9 interrogates students on applying these principles across diverse scenarios: predicting pH shifts in titrations, calculating baseline pH from known acid or base concentrations, and interpreting logarithmic transitions. For instance, when diluting a strong acid like HCl, pH rises sharply, drastically reducing [H+]—a shift mathematically predictable through logarithmic decay.
Conversely, adding hydroxide ion to a solution lowers pOH, increasing pH and deprotonating species critical in biochemical pathways.
Core Equation: The Bridge Between pH and pOH
The central equation, pH + pOH = 14, is not merely a formula but a diagnostic tool. It anchors all calculations and validates results: - If pH = 3, pOH = 11; - If pH = 11, pOH = 3.This direct relationship enables rapid checks: a measured pH of 2.7 suggests a strongly acidic solution (pOH = 11.3), while a pH of 12.5 confirms alkalinity (pOH = 1.5). Such precision is indispensable in industrial quality control, pharmaceutical formulation, and environmental monitoring, where even minor deviations can disrupt processes or compromise safety.
Beyond arithmetic, the answers emphasize conceptual mastery.
Understanding that pH reflects [H+] guides correct interpretation of solution behavior: low pH means high acidity and potential corrosiveness, while high pH indicates alkaline conditions that may precipitate metals or denature proteins. In analytic chemistry, pH determines the endpoint of titrations—indicating when neutralization occurs—relying on characteristic pH changes near equivalence points. Moreover, in biological systems, pH homeostasis is critical: enzyme activity, oxygen transport, and cellular integrity all depend on narrow pH ranges tightly regulated by physiological buffering.
Real-World Implications of pH and pOH Calculations
Environmental Regulation Water quality standards enforce pH limits to protect aquatic ecosystems. For example, freshwater ecosystems thrive between pH 6.5 and 8.5; deviations beyond this range inhibit fish reproduction and disrupt microbial communities. Regulatory agencies use Problem Set 9-style calculations to assess pollution impact—measuring runoff acidification from industrial discharges or soil leaching—and enforce corrective measures when pH shifts exceed thresholds.Pharmaceutical Design Drug stability and solubility hinge on pH. Many active pharmaceutical ingredients degrade rapidly in alkaline conditions; precise pOH control ensures formulation longevity. By predicting dissolution rates and moisture sensitivity based on pH, chemists optimize dosage forms—from tablets to injectables—enhancing patient safety and treatment efficacy.
Industrial Processes In chemical manufacturing, maintaining target pH stabilizes reactions and prevents unwanted byproducts. For instance, in pulp and paper mills, pH regulation prevents lignin degradation and remedy corrosion. Real-time pH monitoring, informed by Problem Set 9 answers, integrates with automated controls to sustain optimal operating conditions and reduce waste.
The Logarithmic Advantage: Why pH and pOH Resist Linear Thinking
The logarithmic nature of pH and pOH enables management of vast concentration spans within manageable numerical ranges. A solution with 0.000001 M H+ (pH 6) releases a million-fold dilution relative to pure water (pH 7), yet is expressed succinctly. This efficiency empowers scientists to detect trace contaminants—such as heavy metal ions affecting pH via protonation—to sub-picomolar levels, a capability essential in environmental sensing and clinical diagnostics.Ultimately, mastering Problem Set 9’s pH and pOH answers transcends academic exercise—it equips individuals to interpret and influence chemical reality. From safeguarding drinking water to advancing drug development, the ability to calculate, predict, and respond to pH shifts is indispensable. In an era where precision chemistry drives innovation, understanding these logarithmic tools is not optional: it is foundational.
Related Post

Keanu Reeves’ Gut-Wrenching Journey Through Grief: The Tragic Loss of Jennifer Syme That Defined a Man’s Tragedy

Teton Pizza Jackson Wy Unleashes Next-Gen Sauce That’s Redefining Mountain Town Flavor

Nadine Drescher: A Rising Star Redefining Entertainment with Grit, Talent, and Vision
Estarossa: The Enigmatic Demon of Love in Seven Deadly Sins

