Lewis Symbol for Carbon: The Built-in Blueprint of Life and Chemistry
Lewis Symbol for Carbon: The Built-in Blueprint of Life and Chemistry
At the heart of organic chemistry and the molecular foundation of life lies carbon — twice visually manifested in the elegant simplicity of its Lewis symbol: fficiency in flexibility. Unlike elemental symbols that rely solely on standard notation, carbon’s Lewis diagram — ╔═════════¬═╗ — distills the atom’s capacity to forge stable bonds, hold diverse valencies, and enable the structural complexity underpinning biochemistry. The Lewis symbol, a cornerstone of valence bond theory, reflects carbon’s unique electron configuration: six valence electrons arranged to maximize bonding potential through covalent interactions.
Carbon’s atomic number six places its valence shell at openness, allowing it to share, lose, or gain electrons depending on environment — a trait central to its role in thousands of organic compounds. Tracing the Lewis structure ╔═════════╡═—══════╗ reveals carbon paired symmetrically with hydrogen (H) in molecules like methane (CH₄), or covalently linked in carbon chains and rings. This ability to form four stable single bonds stems from carbon’s electron arrangement: \(1s^2 2s^2 2p^2\), leaving room for four electron pairs to attach.
“Carbon’s chemistry hinges on this flexibility,” notes chemists at the American Chemical Society, “allowing atoms to weave networks from simple beginnings into life’s molecular dance.” Carbon’s versatility manifests in multiple valences: it readily forms single, double, and triple bonds — a rarity among elements. With two unpaired electrons in the 2p orbitals, carbon can align with other p orbitals to build σ (sigma) and π (pi) bonds, enabling diverse structures from single bonds in ethane (C–C) to double bonds in ethene (C=C). This bond adaptability explains carbon’s unparalleled role in hydrocarbons, alcohols, carboxylic acids, and aromatic systems.
The Lewis notation simplifies this complexity: the ╔═════════╡═— symbol encapsulates not just state, but dynamic reactivity — oxygen may bond, hydrogen shifts position, carbon remains the central architect. Beyond static structures, carbon’s Lewis symbol reveals its role as a scaffold for higher-order chemistry. In biochemistry, for instance, glucose (C₆H₁₂O₆) unfolds from carbon’s sp³ hybridization, forming rings in cyclical isomers stabilized by precise electron sharing.
Mutations and enzyme interactions manipulate these bonds, all traceable to the atom’s Lewis blueprint. In materials science, carbon’s bonding diversity fuels innovations — graphene’s 2D lattice, diamond’s tetrahedral network, and polymers’ repeating units — each shaped by carbon’s ability to bond powerfully and directionally. “The Lewis structure is carbon’s amino acid,” says a materials researcher, “a silent blueprint for endless molecular innovation.” Carbon’s chemistries — thermal, electrical, structural — all originate from this single symbol.
Whether oxygen shares electrons in CO₂, silicon borrows bonding logic in silicon-based systems, or carbon borrows electron density in catalytic reactions, the foundational rule remains: the Lewis symbol ╔═════════╡═— captures the atom’s bonding identity, its dance through valence shells, and its pivotal role in shaping molecular life. From pharmaceutical design to nanotechnology, carbon’s Lewis representation is more than notational convenience — it is the visual grammar of chemistry’s most vital element, encoding the potential that fuels both nature’s complexity and human innovation. It is, quite simply, the single symbol that binds atoms to ideas, reactions to revelations, and molecules to meaning.



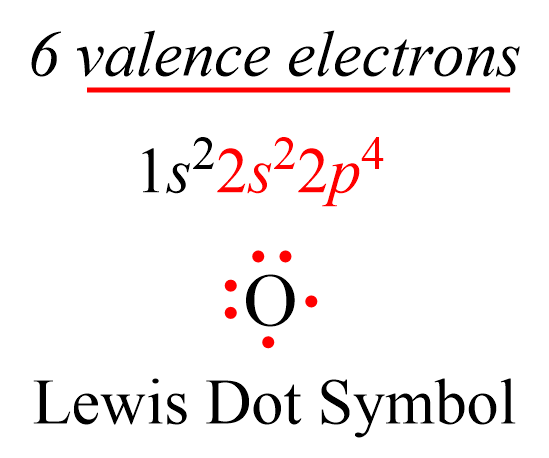
Related Post
Hdhub4Ucontact Your Ultimate Guide To Streaming And Downloading Movies Online

A Multifaceted Persona In The Spotlight: Navigating Complexity with Depth and Nuance

Walmart Tire Center in Martinsburg, WV: Your Complete Tire Solution, Delivered