Hyper-Efficient Shuttles: How Proteins and Lipids Package and Transport Cargo in Exported Vesicles
Hyper-Efficient Shuttles: How Proteins and Lipids Package and Transport Cargo in Exported Vesicles
Vesicles act as cellular couriers, ferrying essential molecules across membranes to sustain organelle communication, nutrient distribution, and waste removal. Central to this process are specialized protein-lipid complexes embedded within the vesicle membrane, which meticulously package cargo, ensure structural integrity, and orchestrate fusion with target membranes. The orchestration of proteins and lipids in exported vesicles is not merely structural—it is a dynamic, highly regulated biochemical dance that underpins cellular logistics.
Understanding the precise roles and packages of proteins and lipids reveals the sophistication of intracellular transport systems.
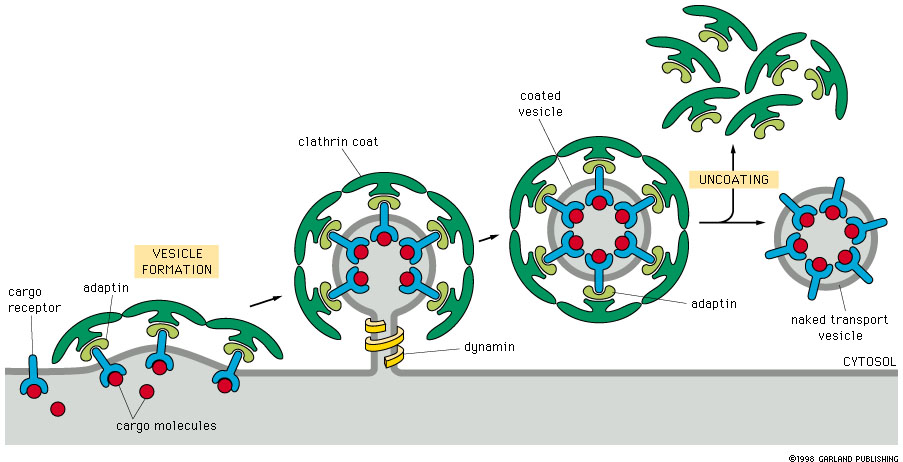
The vesicle formation begins with the selective sorting of cellular cargo—proteins, lipids, and metabolites—into nascent vesicles. Key to this sorting are cargo-binding proteins that recognize specific sorting signals and anchor cargo to coat complexes, such as clathrin, COPI, or COPII.
“Coat proteins act as molecular tethers, defining which molecules are packaged and ensuring fidelity in transport,” explains Dr. Elena Markova, a cell biologist at the Max Planck Institute. “They not only select substrates but also induce membrane curvature, initiating vesicle budding.” These coats are integral components of the vesicle package, forming a protective shell that maintains cargo stability en route.
Lipids, far from being passive structural elements, actively shape vesicle identity and function. The lipid composition—encompassing phosphatidylcholine, phosphatidylethanolamine, sphingolipids, and cholesterol—varies across organelles, influencing membrane fluidity, curvature, and protein recruitment. “Lipid microdomains function as sorting platforms where specific proteins cluster and interact,” notes Dr.
Rajiv Patel, a membrane biophysicist. “For instance, cholesterol enhances membrane rigidity and stabilizes protein complexes essential for fusion competence.” The asymmetric distribution of lipids across the vesicle membrane further fine-tunes signaling and fusion dynamics, enabling vesicles to communicate precisely with target membranes.
Proteins within vesicles serve multifunctional roles—from tethering and transportation to enzymatic modification and fusion regulation.
SNARE proteins, arguably the best-studied, drive membrane fusion through coiled-coil interactions: v-SNAREs on vesicles pair with t-SNAREs on target membranes, pulling the vesicle into close apposition. Beyond fusion, motor proteins like kinesins and dyneins exploit microtubular tracks to guide vesicles along precise cellular highways. “The specificity of SNARE pairing ensures that vesicles dock only at intended destinations,” clarifies Dr.
Sofia Liu, whose research focuses on vesicle trafficking in neurons. “This spatial accuracy is critical for synaptic vesicle release and neurotransmitter delivery.”
Alongside proteins, lipids participate actively in vesicle biogenesis. Enveloping cargo vesicles, lipid-modifying enzymes such as phospholipases and liptransferases alter headgroup composition and fatty acid saturation, tailoring the lipid environment to meet functional demand.
“Lipid remodeling is essential for adapting vesicles to dynamic cellular environments,” says Dr. Markova. For example, phosphoinositides—key signaling lipids—regulate vesicle budding, motility, and fusion independently of cargo, demonstrating that lipid content is not just structural but informational.
To further illustrate the integration of proteins and lipids, consider the secretory pathway: proteins destined for secretion are assembled in the rough ER, sorted at the Golgi via lipid-dependent mechanisms, packaged into clathrin-coated vesicles, and directed to the plasma membrane. Each step involves precise coordination—cargo receptors bind to specific lipids, coat proteins engage lipid-anchored adaptors, and SNAREs ensure fusion with the correct target. This level of orchestration minimizes errors and prevents cross-talk between pathways, a necessity for cellular homeostasis.
Vesicle trafficking is equally finessed in specialized contexts such as endocytosis, autophagosome formation, and exosome release. In each, the protein-lipid cargo package carries distinct signatures and functional payloads. Exosomes—nanoscale vesicles involved in intercellular communication—differ in lipid composition and protein cargo from secretory vesicles, reflecting their role in signaling via lipid-protein complexes that retain bioactive cargo integrity.
The importance of this system extends beyond basic biology—defects in vesicle export mechanisms are implicated in diseases ranging from neurodegeneration to cancer. Disrupted lipid metabolism or SNARE dysfunction can lead to misrouted cargo, impaired signaling, or synaptic failure. Targeting vesicle trafficking proteins and lipid pathways now represents a frontier in therapeutic development, offering precision tools to correct cellular shipping errors.
What begins as a simple vesicle—just a thin lipid shell—is ultimately a marvel of cellular engineering. Proteins and lipids collaborate not as isolated actors but as an integrated complex, each teaming with precise roles to ensure accuracy, speed, and adaptability in intracellular transport. This dual packaging system underpins the cell’s ability to coordinate complex functions with remarkable precision.
As research continues to peel back layers of vesicle biology, it becomes increasingly clear: the proteins and lipids within exported vesicles are not just components—they are the logistics carriers of life’s intricate choreography.



Related Post

Family Fun That Sparks Joy: The Ultimate Guide to Unforgettable Time Together

Post Malone and His New Love: A Closer Look at the Heart Behind the Hype

Tracy Tweed: From Style Icon to Fashion Industry Pioneer – A Legacy Woven in Threads

