Hydrostatic Pressure vs Osmotic Pressure: The Invisible Battle at the Cellular Frontier
Hydrostatic Pressure vs Osmotic Pressure: The Invisible Battle at the Cellular Frontier
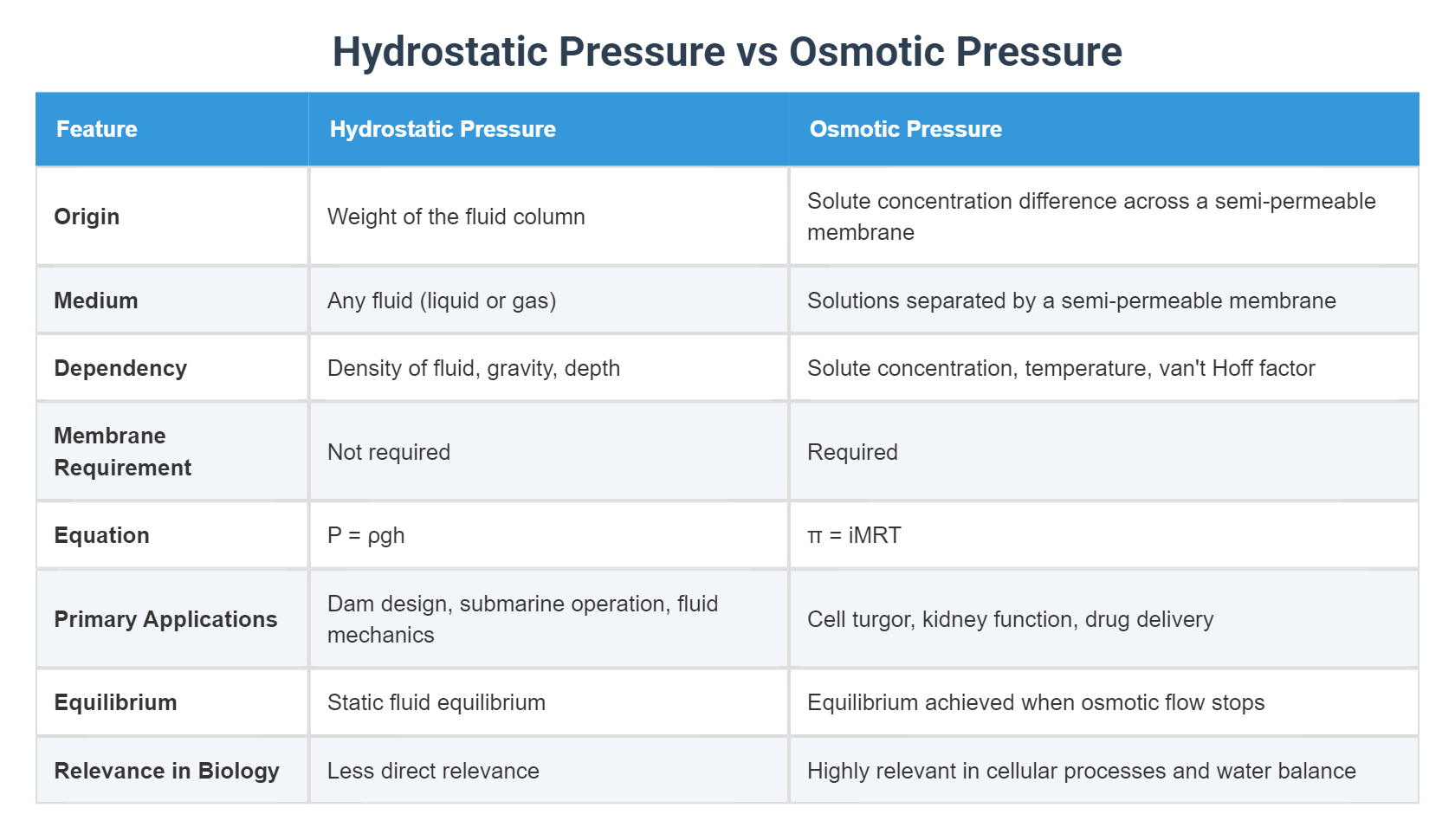
At the intersection of physiology, biology, and physics lies a silent yet decisive struggle: hydrostatic pressure versus osmotic pressure. These opposing forces govern fluid movement across membranes, shaping everything from kidney function to cellular integrity. While hydrostatic pressure drives fluid outward under mechanical stress, osmotic pressure pulls water inward in response to solute concentration gradients.
Understanding their dynamic balance is essential to grasping how life sustains itself at the microscopic and systemic levels. far beyond textbook diagrams, this interplay determines everything from blood pressure to cell swelling—and in medical settings, it can mean the difference between health and crisis.
The Mechanical Force: Understanding Hydrostatic Pressure
Hydrostatic pressure, derived from fluid motion and confinement, is the push exerted by a fluid at equilibrium due to gravity, flow, or compression. In biological systems, it arises from blood pressure pushing against vessel walls, interstitial fluid pressure within tissues, and even osmotic regulation in the kidneys.
“Hydrostatic pressure is essentially the weight and movement of fluid exerting force on surrounding surfaces,” explains Dr. Elena Marquez, a biomedical engineer specializing in vascular dynamics. “In capillaries, the hydrostatic pressure on the arterial side forces plasma and nutrients out into the tissue space.”
Physically quantified in pascals (Pa) or millimeters of mercury (mmHg), hydrostatic pressure reflects the kinetic energy of fluid in motion.
In capillaries, the net hydrostatic pressure typically ranges from 25–35 mmHg, while in venous systems it drops to less than 10 mmHg due to fluid consumption by tissues and absorption back into blood vessels. This gradient governs filtration and reabsorption—the fundamental processes of nutrient delivery and waste removal.
Control of hydrostatic pressure is vital for circulatory and renal function.
When arterial pressure rises, capillary hydrostatic pressure increases, raising the risk of fluid leakage into tissues—swelling known as edema. Conversely, decreased pressure impairs nutrient exchange, compromising cellular metabolism. This dual role makes hydrostatic pressure a cornerstone of fluid homeostasis in the human body.
The Concentration Pull: Unraveling Osmotic Pressure
Osmotic pressure emerges from differences in solute concentration between compartments, driving water movement to equalize solute levels.
Governed by van’t Hoff’s law, osmotic pressure (π) is calculated using the formula π = iCRT, where i is the van’t Hoff factor, C the molar concentration, R the gas constant, and T the absolute temperature. Unlike hydrostatic pressure, which is physical force, osmotic pressure is a chemical potential difference—water’s natural tendency to equilibrate solute exposure.
Within cells, osmotic pressure maintains volume and function.
Intracellular proteins and ions create a high solute environment, drawing water in to prevent shrinkage. “Cells are governed by osmotic balance more than most realize,” notes Dr. Raj Patel, a cell biologist at the Institute of Molecular Physiology.
“A smartphones’ dehydrator effect in hypovolemic states—where blood draws fluid from cells—shows osmosis acting as a protective, yet fragile, buffer.”
In plasma and interstitial fluid, osmotic pressure from proteins (especially albumin) counteracts hydrostatic forces. This delicate equilibrium, described by Starling’s law, ensures fluid neither floods tissue nor vanishes into it. Disruption—such as in severe dehydration or liver failure—shifts the balance, causing dangerous swelling or collapse of circulatory volume.
The competing actions of hydrostatic and osmotic pressure thus form a precise, dynamic regulation system essential to physiological stability.
The Dynamic Equilibrium: When Forces Meet in Biological Systems
The real-world relevance of this pressure war lies in the continuous equilibrium between hydrostatic and osmotic forces, often quantified as net filtration pressure. “Inside healthy capillaries, hydrostatic pressure pushes fluid out, while osmotic pressure—largely from plasma proteins—pulls water back,” explains Dr. Marquez.
“When they balance, no net movement occurs—fluid exchange proceeds efficiently without damage.”
Clinical illustration: in kidney nephrons, this balance enables precise urine formation. As fluid filters through the glomerulus (governed by hydrostatic force), solutes remain, but as it flows through tubules, osmotic gradients reabsorb water and essential solutes. “Without this partnership, fluid retention or dehydration would spiral into organ dysfunction,” Dr.
Patel stresses. “The kidneys are master regulators, constantly sampling and adjusting these pressures like internal barometers.”
Beyond the kidney, this equilibrium shapes educación in cancer treatment and drug delivery. Tumor microenvironments often exhibit altered osmotic pressures due to leaky vasculature and high solute buildup, impairing hydrostatic flow and drug penetration.
Nanoparticles designed to navigate these gradients must account for both pressure types, optimizing delivery to target sites while avoiding off-target accumulation. The balance isn’t static—it shifts with health, disease, and therapeutic intervention.
Real-World Implications: From Swelling to Survival
Misbalance between hydrostatic and osmotic pressures manifests in visible and dangerous symptoms. Edema—swelling from fluid accumulation—may signal heart failure, kidney disease, or liver cirrhosis.
Conversely, hyponatremia—low blood sodium—lowers osmotic pressure, causing cells to swell, particularly in the brain, with potentially fatal consequences.
In surgical and critical care settings, insight into these pressures guides life-saving decisions. Monitoring interstitial pressures helps prevent organ edema during mechanical ventilation.
Rehydration therapies are tailored to restore osmotic balance, often using isotonic solutions to gently draw water into blood without shocking cells.
In space medicine, zero gravity flattens the hydrostatic gradient, disrupting fluid distribution and triggering facial puffiness and leg thinning. Engineers and doctors now simulate gravity’s pull using intraventricular pressure sensors and adaptive fluid management, showing how understanding both pressures shapes technologies for extreme environments.
The Delicate Dance: A Lifelike Homage to Physical Balance
Hydrostatic and osmotic pressures are not abstract forces—they are constant companions in sustaining life.
Their interplay, invisible yet powerful



Related Post
Brian Bosworth & His Wife: Unveiling the Private Life Behind the Hollywood Glamour
Tampa Bay Rays Vs Red Sox Match Player Stats: Deep Dive into Key Performances
What Is Friday? Myth, Meaning, and the Modern Work Week

