How Many Protons Defines Lithium? Unlocking the Secrets of This Light Element
How Many Protons Defines Lithium? Unlocking the Secrets of This Light Element
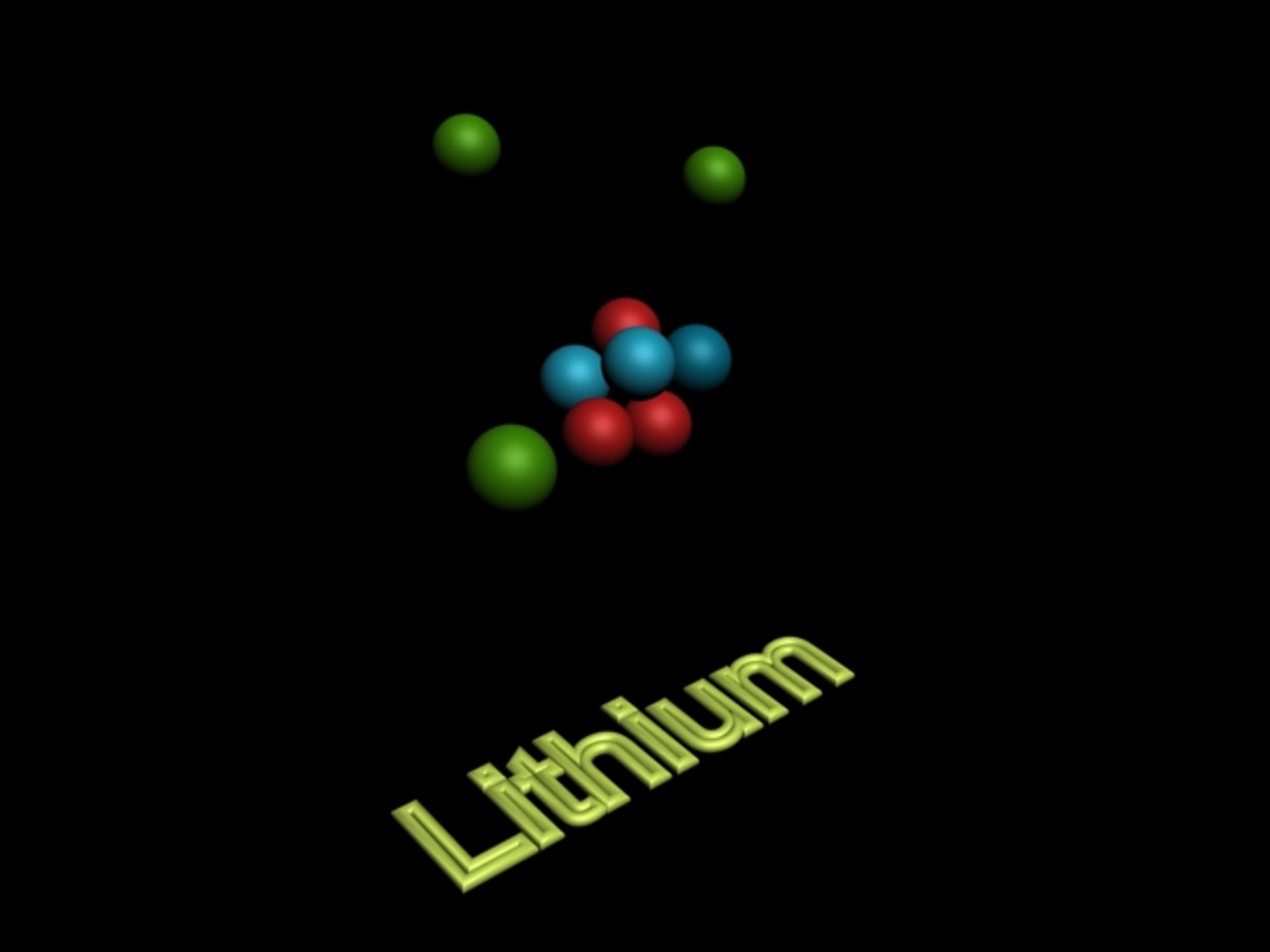
Lithium, the lightweight alkali metal with atomic symbol Li, holds a fundamental property that lies at the heart of its chemical identity: it contains three protons in its nucleus. This single number—three—defines lithium’s place in the periodic table and drives its unique behavior in nature and technology. With an atomic number of 3, lithium’s proton count is not just a static fact—it is the key to understanding its reactivity, fusion potential, and role in modern applications from batteries to medicine.
At the core of every atom is the nucleus, composed of protons and neutrons. For lithium, exactly three protons confirm its position in Group 1 of the periodic table, where alkali metals exhibit singular characteristics such as extreme flammability, high ionization energy barriers, and the ability to lose one electron easily. The presence of three protons directly influences the electronic configuration, chemical bonding tendencies, and nuclear stability of the element.
As noted in foundational atomic theory, "The number of protons constitutes the atomic number, dictating an element’s identity and regulating its physical and chemical behavior." Lithium’s three protons thus anchor its classification and behavior.
Understanding lithium’s proton count is essential to grasping its role in energy storage and advanced materials. Within lithium-ion batteries, lithium ions (Li⁺), stripped of their single positive charge, migrate between electrodes during charge and discharge cycles.
The simplicity of lithium’s atomic structure—three protons, three electrons—enables efficient ion movement with minimal energy loss, a critical factor in battery performance. “Lithium’s monoprotomic nature facilitates rapid ion conduction,” explains Dr. Elena Rodriguez, a materials scientist at the Institute for Advanced Energy Storage.
“Fewer protons mean fewer inner-shell interactions, reducing resistance and enhancing conductivity.”
Beyond consumer electronics, lithium’s proton count has profound implications in nuclear physics. Although lithium-6 and lithium-7—two stable isotopes—have three protons each, their differing neutron counts yield distinct nuclear stability and applications. Lithium-6, used in neutron detection and neutron defense systems, benefits from its low neutron emission under irradiation.
In contrast, lithium-7, with three protons and four neutrons, plays a role in fusion research due to its potential as a fuel in lithium-blown plasma reactions. “The consistency of three protons across isotopes maintains lithium’s versatility,” states nuclear physicist Dr. Rajiv Mehta.
“It allows predictable behavior in both medical isotopes and energy generation.”
In medical applications, lithium compounds such as lithium carbonate are widely prescribed for bipolar disorder treatment. The proton count influences how these ions interact with biological systems—particularly their ability to cross cell membranes and modulate neurotransmitter activity. “The three protons give lithium just enough charge to interact effectively with brain receptors without being overly reactive,” explains Dr.
Sarah Lin, a clinical chemist specializing in neuropsychopharmacology. “This balance underpins its therapeutic efficacy and safety profile.”
Geologically, lithium is rare but increasingly significant. Found in brine deposits and lithium-rich minerals like spodumene, natural lithium results from weathering processes that concentrate this monoprotic element.
Unlike elements with variable proton numbers, lithium’s fixed atomic number ensures uniformity across global deposits. “There’s no variation in lithium’s proton count—only in isotopic distribution,” notes geochemist Dr. Amir Khan.
“This consistency simplifies mining and refinement, enabling scalable production.”
Classified by its atomic number, lithium stands as a quintessential monoprotamic element. The number three defines not only its place on the periodic table but governs its electron dynamics, nuclear characteristics, and practical utility. From powering smartphones to enabling clean energy storage, lithium’s three protons are more than a numerical detail—they are the silent architects of a host of scientific and technological marvels.
Understanding this core atomic trait reveals why lithium remains indispensable in both fundamental science and daily life.


Related Post

GTA Vice City On Mac: A Thrilling Guide

Staring: The Unspoken Language of Eyes That Speaks Louder Than Words
Female Pokémon: Guardians, Innovators, and Icons of a Dynamic Legacy

Nissan Sentra: A Deep Dive Into Its History & Evolution

