H2O Unraveled: The Molecular Bonds That Make Water Unique
H2O Unraveled: The Molecular Bonds That Make Water Unique
At the heart of water’s extraordinary properties lies a simple yet profound secret: its molecular structure and the bonds linking its atoms. Water, H₂O, is far more than just a abundant liquid filling Earth’s surface—it is a dynamic compound shaped by the invisible forces between its hydrogen and oxygen components. The manipulation of these bonds governs water’s ability to dissolve, regulate temperature, support life, and sustain complex chemical reactions.
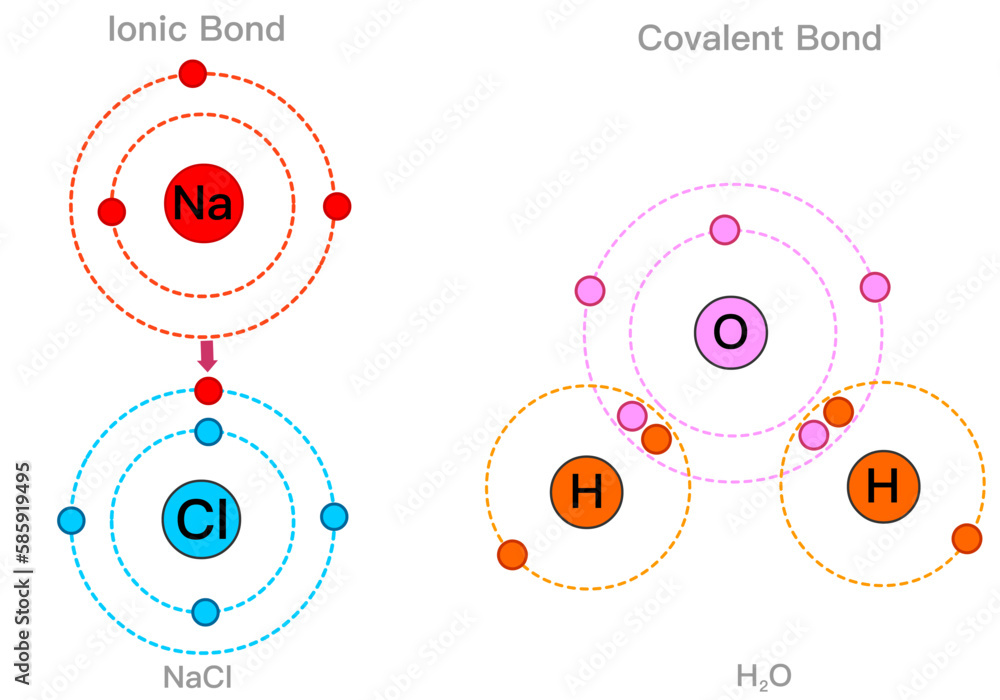
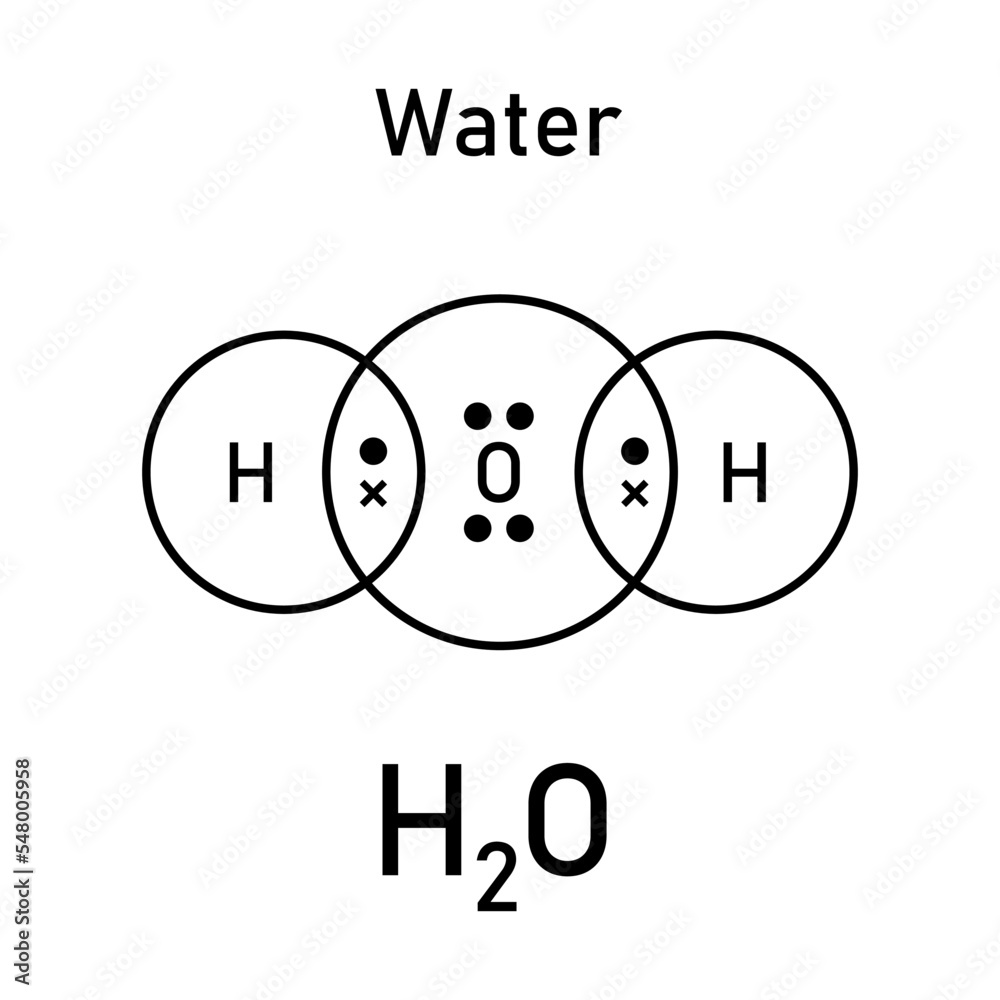
Understanding H₂O’s core bonds reveals why water remains a cornerstone of biology, climate, and industry. Foundations of Water: The Covalent Bond The story begins with covalent bonding, the chemical glue that ties hydrogen and oxygen into a single molecule. Formed when oxygen shares a pair of electrons equally with two hydrogen atoms, each hydrogen-oxygen covalent bond is strong but not unbreakable.
The electron pairs cluster near oxygen, creating a polar molecule—oxygen carries a partial negative charge (δ⁻), while hydrogen carries a partial positive charge (δ⁺). According to Dr. Jane Carter, a physical chemist at MIT, “The oxygen atom’s high electronegativity draws electrons closer, generating a charge separation that defines water’s polarity.” This electron asymmetry is fundamental: it sets the stage for hydrogen bonding, the next critical layer in water’s behavior.
Greater Than the Sum: Hydrogen Bonding in Action While covalent bonds hold atoms together within the H₂O molecule, it is hydrogen bonding—intermolecular forces between water molecules—that unlocks water’s most distinctive qualities. Each water molecule forms up to four hydrogen bonds through electrostatic attractions: the positively charged hydrogen of one molecule attracts the negatively charged oxygen of another. This network yields remarkable properties far beyond those of similar-sized molecules like hydrogen sulfide or methane.
Consider water’s high boiling point of 100°C—well above what its molecular mass alone would predict. “Hydrogen bonding creates strong intermolecular attraction,” explains Dr. Marcus Lin, a materials scientist specializing in liquids.
“Breaking these bonds requires significant energy, explaining water’s unusual thermal stability and resistance to evaporation.” In ice, the hydrogen bond network freezes into a rigid, open lattice, making water less dense than liquid water—a rare exception in nature. This structural quirk sustains aquatic life in frozen environments and regulates Earth’s climate through ice’s insulating properties.
Beyond ice, hydrogen bonds enable water to act as a universal solvent.
“Every polar or ionic substance dissolves in water because the solvent’s electrostatic fields interact strongly with solute particles,” says hydrologist Dr. Elena Ruiz. “Water’s polarity and dynamic bonding make it ideal for facilitating biochemical reactions, nutrient transport, and mineral weathering.”
Electron Dynamics: Why Oxygen Dominates bond Behavior Oxygen’s influence on water’s bonding profile extends beyond its electronegativity.
The atom’s electron configuration enables higher polarizability, allowing oxygen to form stable yet flexible bonds that adapt under environmental stress. This flexibility allows water to adjust to temperature changes and pressure variations without breaking apart—critical for sustaining life across diverse conditions. A key insight comes from spectroscopy: infrared studies reveal hydrogen bond strength fluctuates under hydration, temperature shifts, and even in the presence of solutes.
“Water doesn’t bond uniformly; its connections are constantly forming and breaking,” notes biophysicist Dr. Rajiv Mehta. “This dynamic equilibrium underpins its remarkable adaptability.”
Moreover, hydrogen bonds contribute to water’s high heat capacity.
“Each water molecule participates in multiple bonds, storing and releasing energy efficiently,” explains Dr. Lin. “This ability helps buffer temperature swings in oceans, lakes, and biological systems—stabilizing climates and preserving delicate habitats.”
From Bond to Biology: Water’s Role Across Scales The interplay of covalent and hydrogen bonding creates environments where life evolves.
In cells, water serves as both solvent and reactant. Enzymes rely on water’s polarity to orient substrates; proteins fold accurately only when hydrophilic regions interact favorably with water molecules. Even DNA stability depends on hydrogen bonding between nucleotide bases—for the double helix to form and replicate with precision.
Agriculture, climate science, and medicine all hinge on understanding these molecular interactions. Precision irrigation models rely on water’s cohesion; drug delivery systems exploit hydrogen bonding to target molecules; climate simulations incorporate water’s latent heat dynamics to predict weather and ocean currents.
Engineering Water’s Bonds: Innovation Inspired by Nature The scientific mastery of water’s bonds extends beyond observation into application.
From desalination membranes that filter ions via selective hydration, to nanostructured surfaces that enhance hydrogen-bonding surface areas—engineers increasingly mimic natural water behavior to solve global challenges. “By designing materials that replicate water’s intermolecular functionality,” concludes Dr. Ruiz, “we can develop smarter irrigation, more efficient energy storage, and advanced medical diagnostics.” In lab research, scientists manipulate hydrogen bonding to create synthetic solvents, self-healing materials, and targeted drug carriers—each step guided by the exact nature of water’s atomic connections.
In essence, the bonds in water are not just chemical details—they are catalysts for life, climate stability, and technological progress. The trio of covalent and hydrogen bonds defines water’s identity, driving its indispensable role on Earth and beyond. Understanding these bonds is more than scientific curiosity; it is a foundation for innovation and sustainability.


Related Post

Onett’s Hidden Roots: How a Mountain Town Shaped Earthbound’s Legendary Legacy

How Your Visceral Organs Power Vitality from the Inside Out

Home to Justice: How Legal Aid of Southern Nevada Empowers Vulnerable Communities

