Examples of Chemical Characteristics That Define Reactivity and Behavior Across Substances
Examples of Chemical Characteristics That Define Reactivity and Behavior Across Substances
From the explosive power of sulfuryl chloride to the life-sustaining stability of water, chemical characteristics determine how substances interact, transform, and behave in environments ranging from industrial labs to natural ecosystems. These intrinsic properties—dictated by atomic structure, bonding, and electron dynamics—nonetheless govern everything from combustion efficiency to biological compatibility. By examining key exemplars across diverse chemical classes, a clearer picture emerges of how chemical behavior is fundamentally predictable, yet uniquely nuanced.
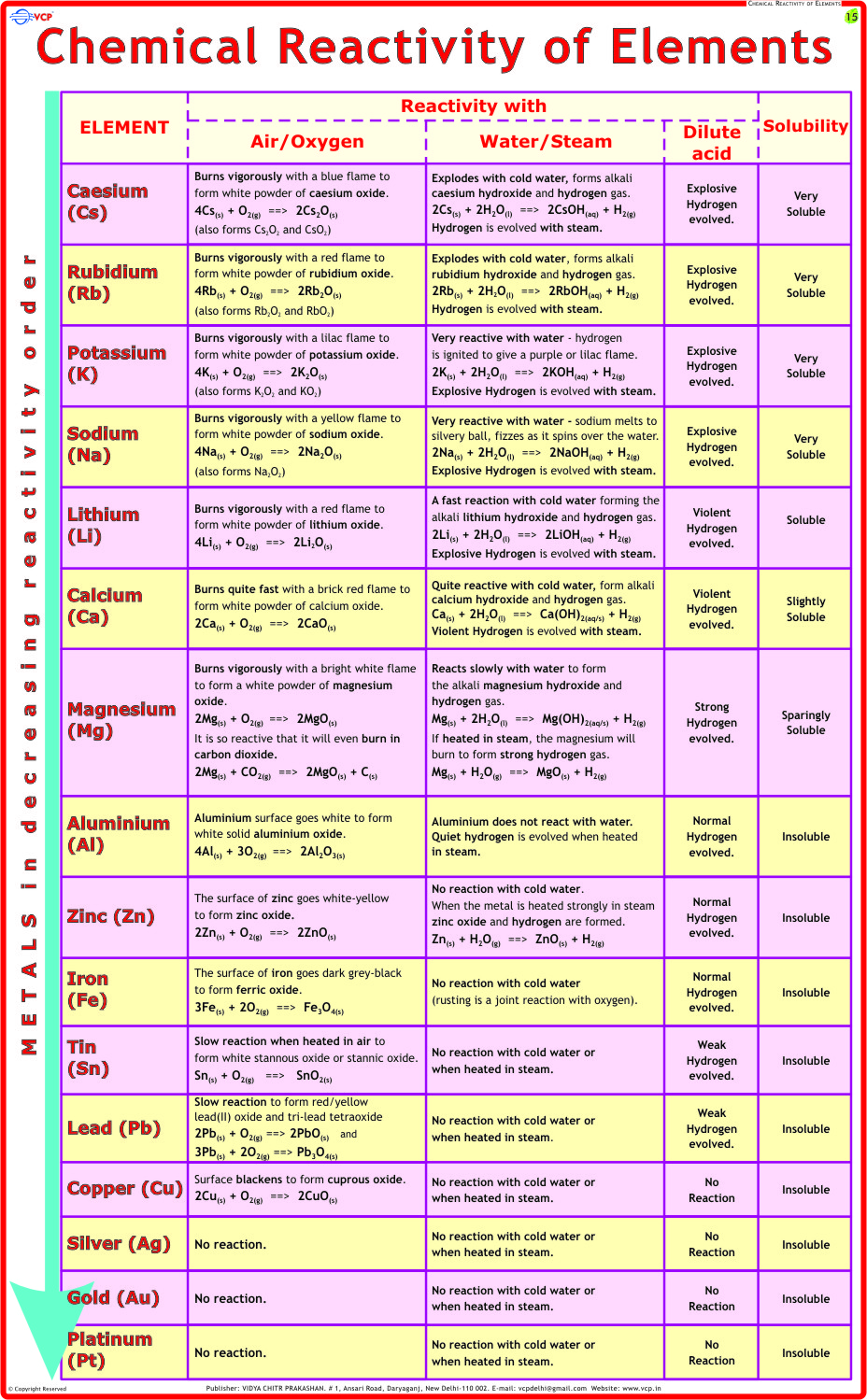
Among the most defined chemical characteristics is reactivity, which determines how readily a substance undergoes transformation. For instance, fluorine exemplifies extreme reactivity as the most electronegative element, readily oxidizing metals and even reacting exothermically with noble gases under certain conditions. Its vigorous interactions stem from a near-absolute electron-attraction tendency, making fluorine a cornerstone in polar synthesis and industrial fluorination.
As chemist Jane Jacobs notes, “Fluorine doesn’t just react—it demands reaction,” encapsulating its commanding role in chemical narratives. In contrast, chlorine, though still reactive, demonstrates controlled industrial behavior—critical in disinfectants and solvents. Intermolecular forces further shape material behavior. Water’s exceptional hydrogen


Related Post

Ten Seconds of Silence, Ten Minutes of Clarity: How ThousandYardStare Redefines Stillness in a Noisy World

Spider-Man’s Dramatic Cast: The Actors Who Shaped The Amazing Spider-Man 2

Imprima O Foguete Da Ketlin: Diversão Garantida!

Springfield, Ohio City Council: Unpacking Party Affiliations and Their Political Impact

