Decoding the Invisible: How C Lewis Dot Structures Revolutionize Chemical Understanding
Decoding the Invisible: How C Lewis Dot Structures Revolutionize Chemical Understanding
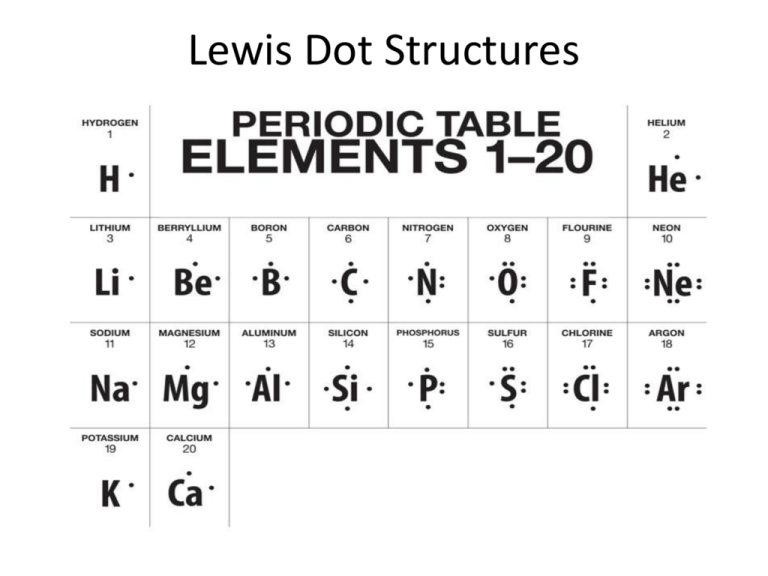
At the heart of inorganic chemistry lies a silent architect shaping molecular behavior—C Lewis Dot Structures. These foundational diagrams, representing valence electrons as dots and lines, transform abstract atomic properties into visual blueprints that define bonding, reactivity, and molecular stability. More than just educational tools, they decode the essence of carbon’s unparalleled role in life and materials, enabling scientists to predict, manipulate, and harness chemical behavior with precision.
The Core of Lewis Dot Structures: Linking Electrons to Function
C Lewis Dot Structures decode complex atoms by focusing on valence electrons—the outermost electrons available for bonding. For carbon, with four valence electrons in its full electron configuration (1s² 2s² 2p²), these dots and lines become a visual language. Here, a single dot represents one electron, and a line symbolizes a bond formed by two shared electrons.The structure conveys not just connectivity, but the geometric and energetic capacity for interaction. Consider carbon’s signature tetrahedral geometry: - Four dots—each representing a valence electron—are arranged around the central carbon atom. - These dots pair via covalent bonds, forming sp³ hybrid orbitals that dictate molecular shape.
- The result: molecules like methane (CH₄), where carbon’s dot pattern enables four identical bonding partnerships, creating symmetrical stability. > "The true power of Lewis structures lies in revealing the invisible dance of electrons—predicting geometry, charge distribution, and reactivity before a single bond is forged." – Dr. Elena Rossi, theoretical chemist, MIT Materials Institute This conceptual framework extends beyond simple molecules.
It applies equally to resonance forms, formal charge calculations, and even complex coordination compounds when paired with electron-pair repulsion models. Yet for carbon—the linchpin of organic chemistry—Lewis structures remain indispensable.
Carbon’s Versatility: From Chains to Cubes—Dot Structures as Molecular Keys
Carbon’s unique tetravalency—its ability to form stable covalent bonds with four others—fuels a staggering diversity of structures.Using Lewis dot diagrams, chemists map every configuration with clarity: - In methane (CH₄): · — single bond by shared pair · · · · with carbon surrounded by four hydrogens in a near-spherical arrangement - In ethylene (C₂H₄): Each carbon shares two pairs with two hydrogens and forms a shared double bond: ·=· — a visual shorthand for one sigma and one pi bond - In diamond (carbon lattice): A 3D network where each carbon bonds tetrahedrally to four neighbors, illustrating how linear dot diagrams scale to extended solids The pattern is universal: each carbon atom’s dot configuration reveals not just its bonding potential, but its role in creating macroscopic phenomena—from flexible polymers to rigid crystalline frameworks. This predictive clarity accelerates discovery, reducing trial and error in synthesis and material design.
Resonance, Formal Charge, and the Limits of the Traditional Dot Model
No discussion of Lewis structures is complete without addressing their conceptual evolution—particularly resonance and formal charge.While basic structures depict single-bond arrangements, real molecules often exhibit electron delocalization. For example, benzene’s stability arises from resonance, depicted through alternating single and double bonds in a circle of alternating dashed lines—acknowledging electron movement not shown in static dot diagrams. Formal charge analysis enhances accuracy: - Calculated as: valence electrons − (non-bonding electrons − bonding electrons)/2 - Guides the choice of lowest energy structure, minimizing formal charge differences Yet these refinements do not negate Lewis structures—they complement them.
They provide a flexible foundation, adaptable through resonance and charge balancing, preserving the model’s utility across organic, inorganic, and materials chemistry.
Applications Beyond Textbooks: From Drug Design to Nanotechnology
Modern chemistry leverages Lewis structures not just in education, but in real-world innovation. Pharmaceutical researchers map drug-molecule interactions by analyzing target proteins’ Lewis-compatible bonding sites.In semiconductor development, graphene and carbon nanotubes’ electronic properties emerge from precise carbon-bond arrangements revealed through dot-based modeling. For instance, the design of carbon-based catalysts—like those mimicking enzyme active sites—relies on tuning carbon’s hybridization and local electron density, visualized early on through dot structures. “Each dot tells a story—of potential bonds, electrons waiting to move, and reactions waiting to unfold,” explains Dr.
Rajiv Mehta, computational chemist at Stanford Quantum Institute. This predictive power accelerates discovery cycles. What once required months of trial and error now unfolds in weeks of structural modeling, guided by the clarity of Lewis dot blueprints.
The Enduring Relevance of C Lewis Dot Structures in Modern Chemistry
C Lewis Dot Structures remain vital not because they replace advanced computational methods—but because they distill complexity into clarity. They provide an intuitive, universal language for understanding how carbon and other elementos engineer the molecular universe. From foundational organic chemistry to cutting-edge nanomaterials, their role is both foundational and far-reaching.As science pushes environmental boundaries—developing biodegradable polymers, efficient solar cells, and novel pharmaceuticals—the clarity offered by these diagrams empowers researchers to envision and build the next generation of materials. More than diagrams, they are living cartographies of electronic possibility, lightning rods for innovation, and irrefutable proof of carbon’s central place in chemistry’s past, present, and future.



Related Post

Unveiling Ted Danson and Kirstie Alley’s Enduring Legacy: Beyond The Cheers Legacy

Matt Berry: The Acting Trios, Comedy Master, and Multi-Genre Powerhouse
The Unspoken Rules of Dark Humor: Discover The Best Dark Jokes For A Twisted Sense Of Humor & 40 Memes That Are Hilariously Relatable
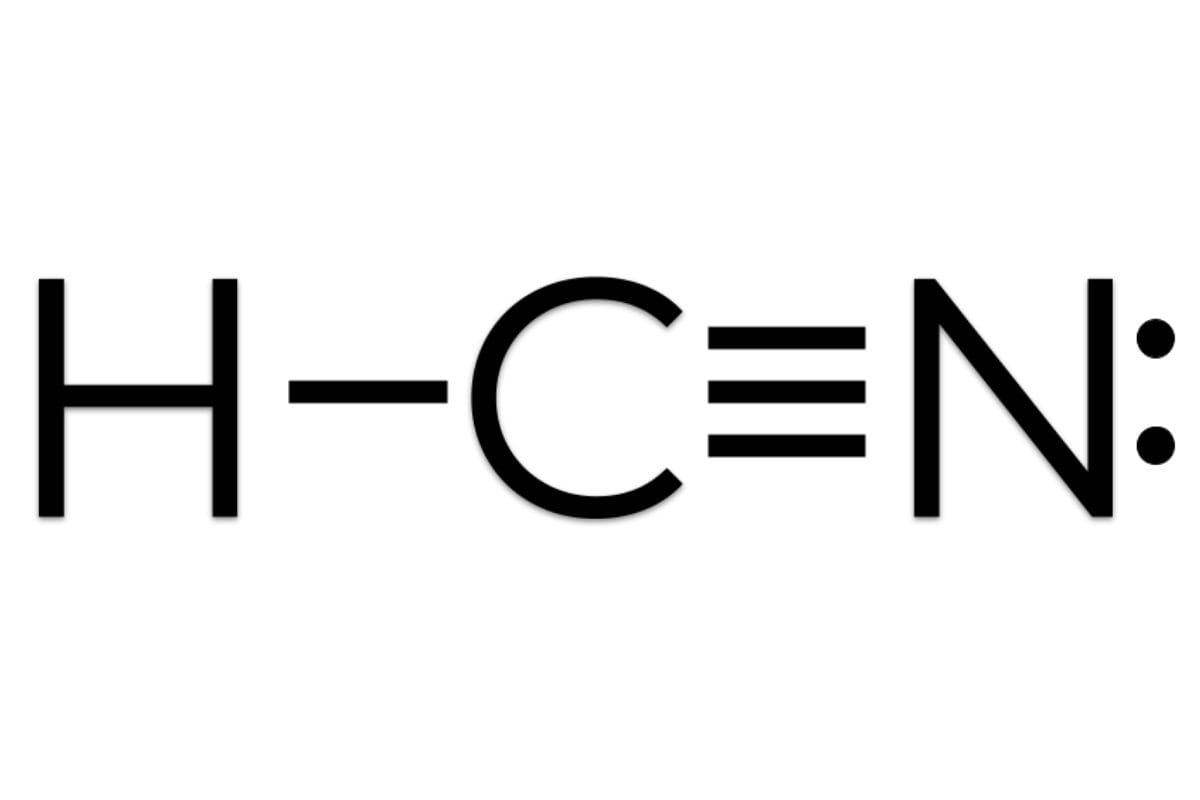
Unlocking the Mystery of Hydrogen Cyanide: Decoding Its Lewis Structure and Reactive Nature

