Decoding Sugar Architecture: Mastery of Glycosidic Bond Fischer Projection in Biochemistry
Decoding Sugar Architecture: Mastery of Glycosidic Bond Fischer Projection in Biochemistry
At the molecular frontier of carbohydrates, the precise orientation of glycosidic bonds dictates the function and fate of complex sugars—sugar structures central to everything from cellular signaling to drug design. The Fisher projection, a carbon-centric labeling system, serves as a visual anchor in deciphering these stereochemical relationships, enabling scientists to map glycosidic linkages with unerring accuracy. By systematically representing hydroxyl groups at specific axial and equatorial positions, the Fischer projection platform empowers researchers to interpret enzyme-catalyzed transformations, predict glycan behavior, and engineer novel therapeutics, transforming abstract sugar geometries into actionable biochemical blueprints.
Glycosidic bonds—covalent links joining monosaccharides via their anomeric carbon—define the three-dimensional architecture of glycans, polysaccharides, and glycoconjugates. The stereochemistry of these bonds—classified as α or β and anchored in specific ring conformations—governs biological recognition events, nutrient metabolism, and pathogenic interactions. Mastery of glycosidic bond orientation is essential: a single bond inversion can alter immunogenicity, enzymatic susceptibility, or binding affinity.
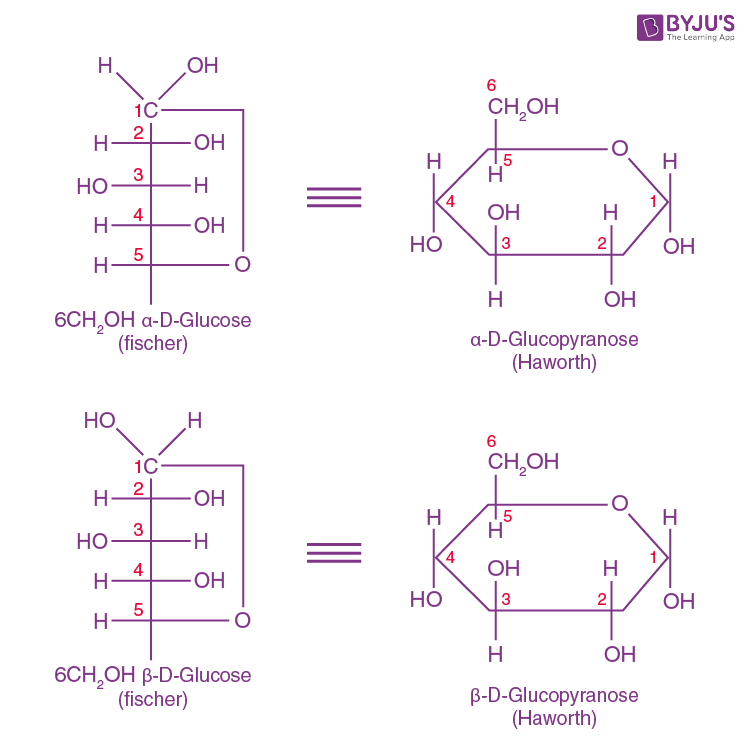
The Fisher projection, originally devised in the 19th century for carbohydrate structure, remains indispensable for visualizing these stereocenters. Unlike other depictions, it preserves relative configurations through consistent labeling of axial (vertical) and equatorial (horizontal) substituents, offering a reversible lens to assess stereochemical equivalence.
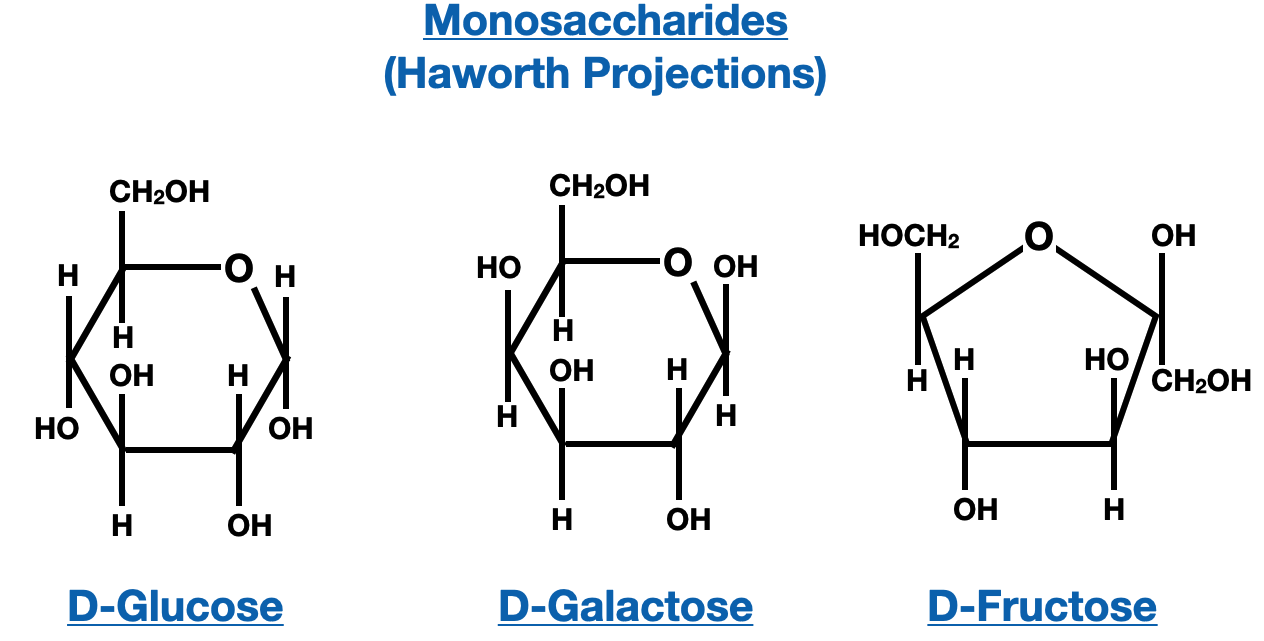
The core design of a Fischer projection for glycosidic bonds hinges on precise notation: horizontal hydrogen atoms project toward the viewer, while vertical hydrogens recede behind, and anomeric hydroxyls are conventionally marked at arbitrary or fixed positions (commonly top for α risers when priorities are clear). Each monosaccharide unit is laid flat, with the anomeric carbon (C1) oriented vertically to serve as a zero reference.Adjacent rings are drawn with axial substituents ascending from top to bottom or vice versa—consistent with chair or boat conformations—while glycosidic linkages appear as horizontal single bonds (C1–C1’) oriented across the plane, rigidly defining α or β stereochemistry. “This method transforms three-dimensional complexity into a two-dimensional language understood across genetics, medicinal chemistry, and glycobiology,” notes Dr. Elena Petrova, a carbohydrate chemist at the Max Planck Institute for Chemical Biology.
Breaking down the structural mechanics, Fischer projections encode critical data: the saccharide’s ring form (pyranose, furanose), anomeric configuration (α or β), and bond linkage type. For instance, a β-D-glucopyranose cathing a α-D-mannose at C1 forms a glycosidic bond classified as Mek焜leikfter → formed as a β-1,4 linkage, depicted as a horizontal bond crossing at the anomeric position with an upward-pointing hydroxyl熊熊熊熊熊熊 → المو演染ả launch. Such specific arrangements are non-negotiable in synthetic biology, where glycosidic orientation determines whether a glycopeptide elicits therapeutic benefit or immune rejection.
The projection also aids in predicting selectivity of glycosyltransferases—enzymes that attach sugars with high positional fidelity—and in reverse-engineering glycoconjugate structures from experimentally derived spectra. Historical adaptation of Fischer notation—originally designed for sugars in the 1880s—has enabled generations of researchers to map enzymatic pathways, design glycomimetics, and explore evolutionary conservation of glycosylation patterns in health and disease.
Beyond static diagrams, glycosidic bond Fischer projections serve as dynamic tools in modern research. Computational glycobiology platforms render these projections in 3D avatars, overlaying experimental data from NMR and X-ray crystallography to validate stereochemical assignments.“We use Fischer-like schemas to visualize linker conformations before solid-state modeling,” explains Dr. Rajiv Mehta, a glycoengineering lead at MIT. “The clarity it offers accelerates rational design of glycotherapeutics targeting cancer or autoimmune disorders.” Furthermore, the notation standardizes communication across disciplines: a Fischer sketch shared between a biochemist and a pharmaceutical chemist conveys precise stereochemistry without ambiguity.
This shared visual syntax lowers cognitive barriers, enabling collaborative breakthroughs in an otherwise opaque domain.
Despite digital advances, the Fischer projection remains foundational. It offers an accessible entry point into stereochemistry when instrumental data is limited, supports rapid hypothesis testing, and anchors foundational curricula in organic and carbohydrate chemistry.
In an era overwhelmed by complex structural datasets, the projection’s simplicity is its strength—transforming chaos into clarity, confusion into interpretable patterns. It bridges empirical discovery with theoretical prediction, making it not just a diagrammatic convention but a cognitive scaffold for mastering sugar biochemistry.
The Structural Legacy: How Glycosidic Bondstellences Shape Biological Function
The precise orientation of glycosidic bonds dictates how glycans interact with proteins, receptors, and pathogens. For example, blood group antigens—determined by subtle sugar linkage differences—trigger transfusion reactions; a single hydroxyl group inversion alters immunogenicity, a finding directly interpretable through Fischer projections.Widely used in glycoprotein glycosylation mapping, these projections help annotate structures that influence drug absorption, microbial adhesion, and immune modulation. In vaccine design, they guide antigenic glycan display, ensuring immune responses target conserved immunogenic epitopes. By codifying stereocenters in a reproducible format, Fischer projections empower scientists to dissect molecular identity, immunity, and disease mechanisms with unprecedented precision.
Standardized application of Fischer notation ensures consistency across global research. Journals, databases, and educational materials rely on consistent iconography—vertical bonds for stereocenters, horizontal lines for glycosidic linkages—enabling rapid cross-validation. In cutting-edge fields like glycoimmunology and synthetic glycobiology, this consistency accelerates discovery.
As biochemistry ventures deeper into the glycome—the vast repertoire of biological sugars—Fischer projections endure as both historical legacy and modern essential, making the invisible spatial architecture of sugars visible to the scientist’s eye.
Ultimately, the Fischer projection of glycosidic bonds transcends mere illustration: it is a structural language that decodes the very grammar of carbohydrates. Its enduring relevance lies not just in its simplicity, but in its power to render three-dimensional complexity navigable, tangible, and actionable—transforming sugar linkages from biochemical curiosities into foundational elements of biological innovation.


Related Post
Russia-Ukraine War: Latest Developments Reshape Global Security Landscape
Uncovered: The Exceptional Ascent of Thespian Jin Ki Joo

The Ninja Mind: Lessons from Lloyd in The Lego Ninjago Movie
Liam Neeson’s Star Power: A Unique Charisma That Defies Trends and Endures Across Decades