Decoding Chemistry’s Blueprint: Mastering the Ocn-Lewis Structure for Precise Bonding Insights
Decoding Chemistry’s Blueprint: Mastering the Ocn-Lewis Structure for Precise Bonding Insights
The Ocn-Lewis structure stands as a cornerstone in chemical education and practice—a meticulously detailed representation that reveals how oxygen and carbon atoms form stable molecules through electron sharing. More than just a diagrammatic sketch, this structure deciphers the invisible dance of electrons, offering clarity on molecular bonding, geometry, and reactivity. For students, researchers, and educators alike, understanding the Ocn-Lewis framework for O–C bonds is not just academic—it’s fundamental to predicting molecular behavior in everything from biological systems to industrial synthesis.
At its core, a Lewis structure is a schematic that illustrates valence electrons and their arrangement around atoms, governed by the octet rule: atoms bond to achieve eight electrons in their outer shell, mimicking the electron configuration of noble gases. Oxygen, with six valence electrons, and carbon, with four, form a unique partnership—often explored through varying Ocn-Lewis structures—where shaped orbits dictate how molecules assemble. Unlike simplistic representations, the Ocn-Lewis model accounts for electron distribution, formal charges, and resonance, providing a nuanced view of molecular stability.
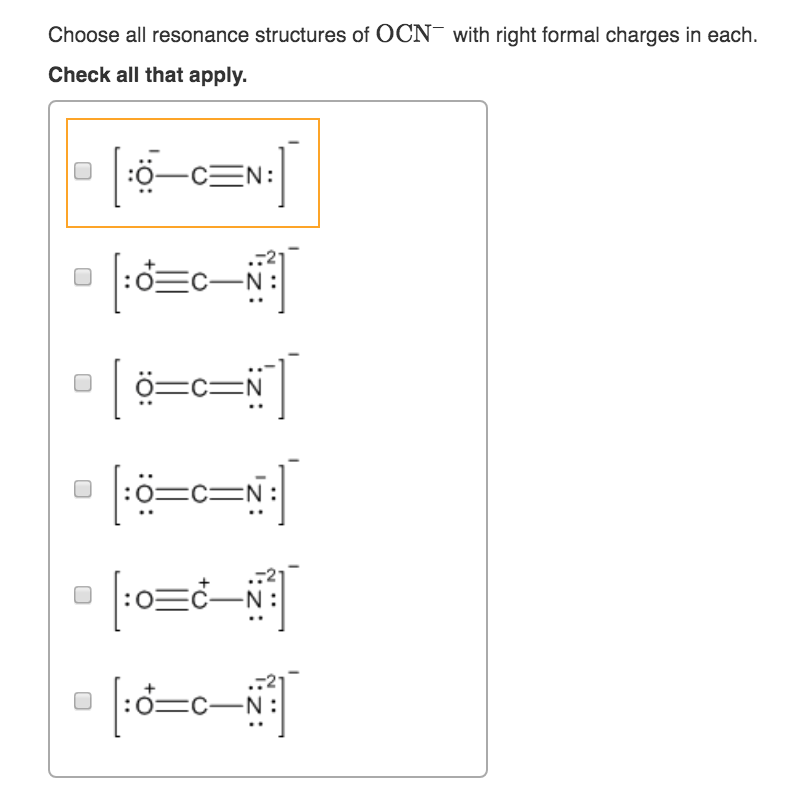
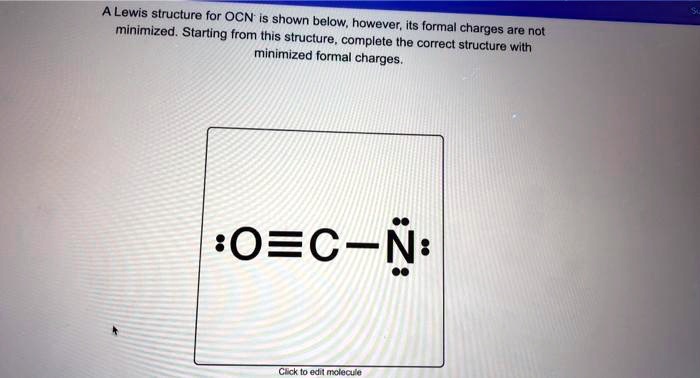
Analyzing the Ocn-Lewis structure of carbon-oxygen composites reveals critical bonding patterns. In molecules such as carbon monoxide (CO) and carbon dioxide (CO₂), oxygen and carbon form distinct configurations—key differences that shape their chemical identity. For instance, CO features a triple bond between O and C, with localized electron sharing that enhances its stability and role as a vital cellular signaling molecule. In contrast, CO₂ presents a linear arrangement with two double bonds, reflecting symmetric electron distribution and one of nature’s most abundant greenhouse gases. Constructing these structures begins with total valence electrons: carbon contributes four, oxygen six, resulting in ten electrons total for CO. Drawing the central carbon atom, preliminary placement of single bonds uses four electrons, leaving six unassigned. These remain to pair around terminal oxygen atoms—each oxygen must complete its octet. Strategic adjustment via double bonds increases electron density between O and C, ensuring atom stability. The resulting Lewis structure for CO—O=C–O—exemplifies efficient electron economy, directing, "Every bond here is purposeful." What distinguishes the Ocn-Lewis structure is its ability to expose subtle electronic features invisible to the naked eye. By assigning formal charges, chemists assess bond polarity and reactivity. For example, CO exhibits a slight dipole due to oxygen’s higher electronegativity, despite nearly symmetric structure. In CO₂, evenly distributed charges reflect linear symmetry, minimizing net dipole. This insight guides predictions of solubility, interaction with enzymes, and environmental impact. Resonance further enriches the interpretation. While isolated Ocn-Lewis forms depict discrete bonds, molecular reality often involves resonance—electron delocalization that stabilizes structures. In advanced models, partial double-bond character across carbon-oxygen links illustrates this dynamic sharing. Though simple 2D diagrams imply static arrangements, Ocn-Lewis foundations lay the groundwork for understanding resonance hybrids crucial in spectroscopy and catalysis. The Ocn-Lewis framework extends far beyond classroom exercises, powering breakthroughs across scientific frontiers. In drug design, predicting how oxygen and carbon bond within molecules informs the stability and bioavailability of therapeutic agents. Carbon monoxide’s toxic affinity for hemoglobin—the O₂ mimic at iron sites—finds clarity through its robust O=C–O geometry, influencing medical treatment strategies. In atmospheric science, CO₂’s persistent linear structure underpins global carbon cycle models, while its vibrational modes, rooted in O-C electron distribution, drive climate change analytics. Even in materials science, Ocn-Lewis principles shape innovation. Functional groups involving O–C bonds—such as carbonyls in aldehydes and ketones—enable synthesis of polymers, pharmaceuticals, and specialty chemicals. The structure’s ability to highlight lone pairs and bonding orbitals aids in tailoring reactivity, guiding precise chemical transformations. Misinterpretations often arise from oversimplifying Lewis structures—for example, assuming only single bonds exist when resonance or electron delocalization is at play. Advanced learners must recognize that the skeletal frame is a guide, not a final truth. Formal charge calculations, though helpful, can mislead if applied blindly; a nuanced approach weighs bond order, overlap, and electronegativity differences. The Ocn-Lewis model, when paired with VSEPR theory, reveals true molecular geometry—bent, linear, or trigonal—ultimately explaining reactivity and physical properties. As research evolves, the Ocn-Lewis structure retains enduring relevance, not as an outdated relic but as a dynamic tool enhancing molecular literacy. Its combination of simplicity and depth makes it indispensable: a bridge between quantum complexity and tangible chemical insight. Whether diagnosing molecular behavior or designing novel compounds, mastering this structure empowers scientists to see beyond symbols into the very architecture of matter—atom by atom, bond by bond. In the intricate choreography of chemistry, the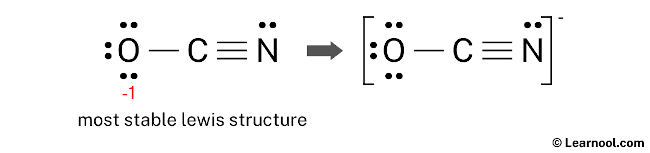

Related Post
US vs Russia: How a Hypothetical Military Showdown Would Unfold Across Battlespaces
Gospel Icons: Trailblazers Who Chase Timeless Inspiration Across Centuries

What Really Happened in the Chris Chan Story: From Faith to Doubt and Back Again

Secrets to Alina Habbas’ Net Worth: The Untold Story Behind Her $50M Minncil Success

