Decoding Acid-Base Reactions: The Power of Weak Acid-Strong Base Titration
Decoding Acid-Base Reactions: The Power of Weak Acid-Strong Base Titration
In laboratory chemistry, precision defines discovery—and nowhere is this more evident than in weak acid strong base titrations, where exact measurements unlock critical insights into molecular behavior. Using a classic example—acetic acid (a weak acid) reacted with sodium hydroxide (a strong base)—researchers harness titration curves to pinpoint equivalence points with remarkable accuracy. This method, foundational in analytical chemistry, serves not only to determine concentration but also to decipher reaction stoichiometry in complex buffered systems.
Through careful calibration and data interpretation, weak acid–strong base titrations reveal the hidden dynamics of proton transfer and equilibrium shifts.
The Chemistry Behind the Titration: Weak Acid vs. Strong Base Dynamics
At the heart of any titration lies the interaction between a weak acid and a strong base—a reaction governed by dissociation constants and proton availability. Acetic acid (CH₃COOH), a weak acid, only partially ionizes in water, defined by its acid dissociation constant \( K_a = 1.8 \times 10^{-5} \).
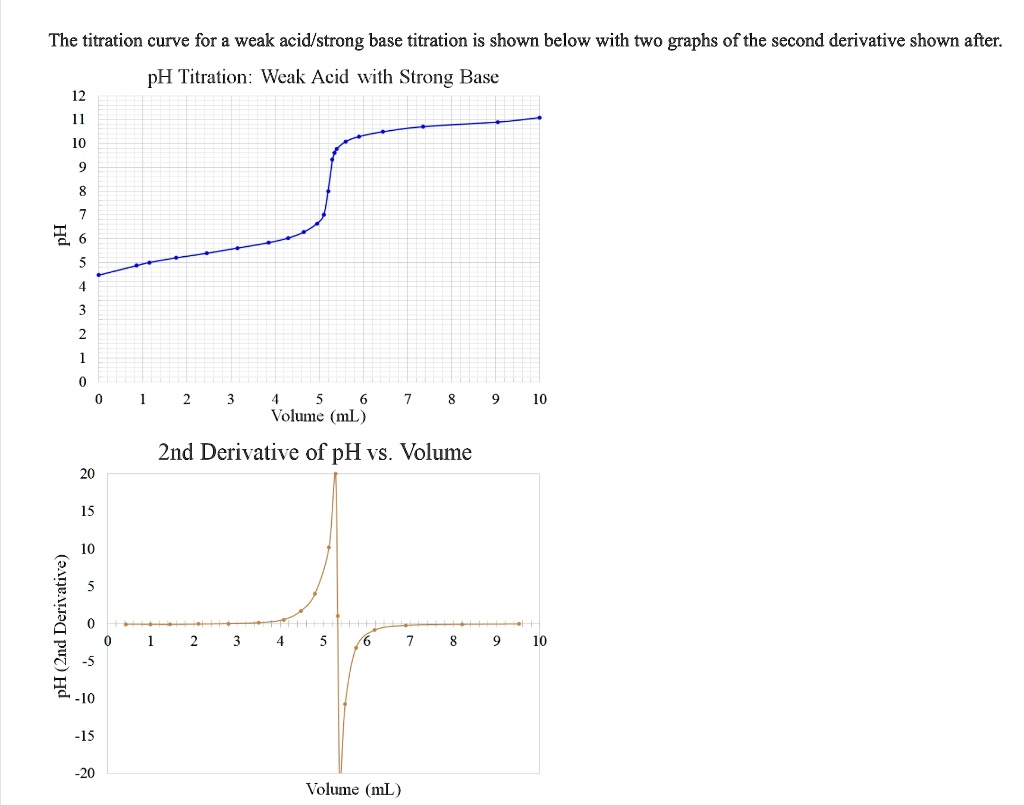
This limited ionization means titration curves exhibit a gradual pH rise until equivalence is reached, followed by a sharp jump. In contrast, sodium hydroxide (NaOH), a strong base, fully dissociates: NaOH → Na⁺ + OH⁻ “complete dissociation ensures a predictable and steep endpoint,” explains Dr. Elena Martinez, analytical chemist at the National Standards Lab.
“disrupting the delicate balance of weak acid equilibria requires precision. This fundamental difference shapes how titration data is analyzed. With weak acids, the buffer region—where pH changes slowly—provides critical pH data before neutralization.”
Step-by-Step: Riding the Equivalence Curve One Drop at a Time
Executing a weak acid–strong base titration follows a systematic protocol designed to capture every stage of the reaction: - **Solution Preparation**: A known concentration of acetic acid solution is transferred to a burette, while a standardized sodium hydroxide solution flows into a large Erlenmeyer flask. - **Initial Measurement**: A pH meter, calibrated with standard buffer solutions, records the starting pH—typically around 2.5 for 0.1 M acetic acid.
- **Titrant Addition**: NaOH is added incrementally—usually 0.1 mL at a time—stirring thoroughly between each addition to ensure mixing. - **Equivalence Detection**: The pH rises sharply near equivalence, signaled by a sudden jump from 2.7 to 8.5 in 0.5 mL increments. - **Data Record and Curve Analysis**: Each titration point—volume, pH, and calculated moles of acid and base—is plotted to generate a titration curve.
Analysts rely on indirect methods to determine the equivalence point, as the pH change lacks a distinct inflection compared to strong acid–strong base titrations. Common approaches include: - **First Derivative Method**: Differentiating the pH curve reveals a pronounced peak at equivalence. - **Linear Ramp Ratio**: Plotting \([NaOH]/[HA]\) vs.
volume shows a linear segment across equivalence, with endpoint at the curve’s steepest rise. These techniques ensure reproducibility, critical in fields like environmental testing and pharmaceutical analysis.
Data in Action: Calculating Concentrations from Equivalence
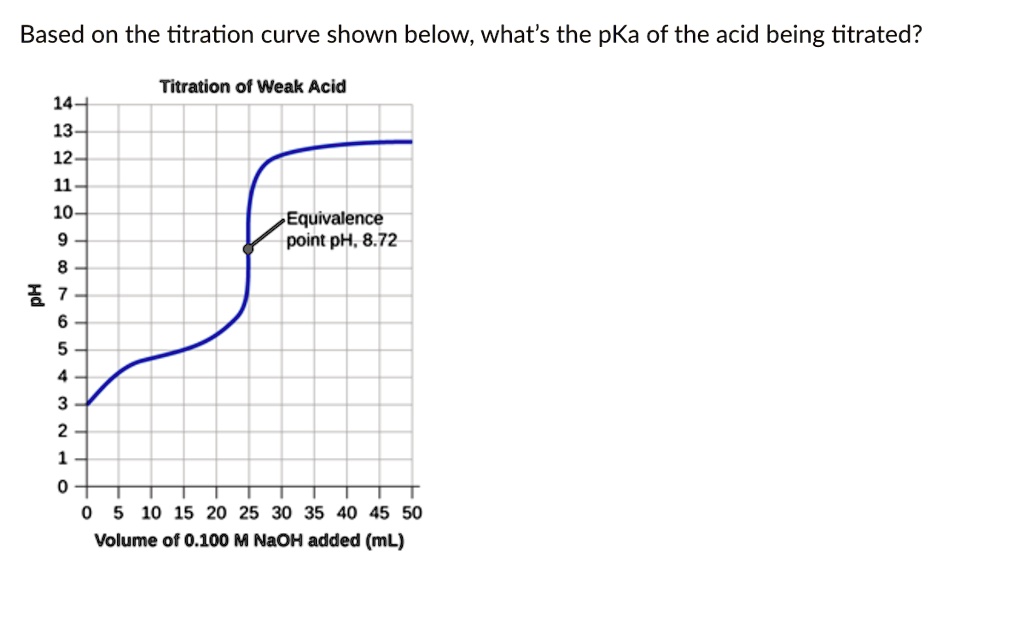
Once equivalence is identified, stoichiometry reveals key concentration data. For acetic acid (monoprotic) titrated with NaOH: \( \text{moles of NaOH} = \text{moles of CH}_3\text{COOH} \) From known volume and molarity of NaOH, and using the equivalence point volume, concentration is derived: \( M_{hab} = \frac{M_{HOH} \times V_{HOH}}{V_{eq}} \) “This equation transforms volume and molarity into molecular precision,” notes Dr.
James Okafor, a physical chemist. “it turns a measured reaction into quantitative insight”.
Consider a real scenario: a 25.0 mL sample of 0.050 M acetic acid is titrated with 0.100 M NaOH.
The equivalence volume is 25.0 mL, yielding: \( M_{hab} = \frac{0.100 \times 0.025}{0.025} = 0.100\ \text{M} \). This standard supports quality control in vinegar manufacturing and buffer formulation in biochemical assays.
The Role of Weak Base and Buffers: Navigating the pH Maze
Weak acid–strong base titrations become even more instructive when applied to buffer systems. Combined with a weak base like ammonia (NH₃), acetic acid forms CH₃COO⁻ and NH₄⁺—a buffer resisting pH swings.
Titration of such a buffer reveals the buffer capacity: the volume of strong base needed to push pH beyond the buffer’s effective range (often pH 6–8).
“Buffers are nature’s stabilizers,”
“and titration quantifies how much acid or base a system can absorb before pH shifts drastically,” says Dr. Priya Kapoor, a biochemical modeller.These investigations underpin drug delivery systems, salmonellosis research, and industrial fermentation, where maintaining precise pH is nonnegotiable.
Conclusion: Mastery in Materia Crвания — The Titration of Weak Acids Defines Clarity
The titration of weak acids with strong bases stands as a cornerstone of analytical rigor—bridging theory and practical precision. Through systematic addition, equilibrium analysis, and curve interpretation, chemists extract exact molarities, identify reaction endpoints, and validate buffer systems.
Far from a mere academic exercise, this method fuels advancements in environmental monitoring, pharmaceuticals, and biochemical engineering. In every calculated drop, we find not just data—but a deeper understanding of the invisible forces that shape matter.


Related Post
Scott Steiner Ready to See Bron Breakker on the WWE Main Roster

Jadwal FOMC Meeting: Kapan & Apa Yang Perlu Diketahui – Motian Ekstrem Bagi Pasar Global
Exploring the Essence: The Core of Aesthetic Appeal

