Decode Chemical Bonds Like Never Before: The Power of Lewis Dot Structure for Nitrogen
Decode Chemical Bonds Like Never Before: The Power of Lewis Dot Structure for Nitrogen
Molecular motion, atomic interactions, and the invisible forces shaping reactions all hinge on precise electron arrangements—and nowhere is this clearer than in Lewis Dot Structure for nitrogen. For one of the most abundant and versatile elements in nature, mastering its dot structure reveals not just connectivity, but reactivity, stability, and function. From biochemistry to industrial chemistry, nitrogen’s electron configuration, illustrated through detailed Lewis Dot Structures, offers a foundational lens through which chemists predict molecular behavior and reactivity.
Nitrogen’s Electron Configuration: The Core of Its Chemical Identity
Nitrogen, with atomic number 7, possesses five valence electrons—two from the 2s orbital and three from the 2p orbitals—making it quintessential to organic chemistry and life itself.
Unlike many elements, nitrogen exhibits strong convergence on stable electron arrangements using the iconic Lewis Dot Structure, a simple yet profound visual tool. Its structure reflects a quest for valency fulfillment through electron sharing or transfer, underpinning its role in ammonia, amino acids, and nitrogen-based fuels. “Nothing about nitrogen’s chemistry is accidental,” notes Dr.
Elena Rodriguez, a physical chemist at MIT. “Its dot structure reveals why it’s both indispensable and selective.”
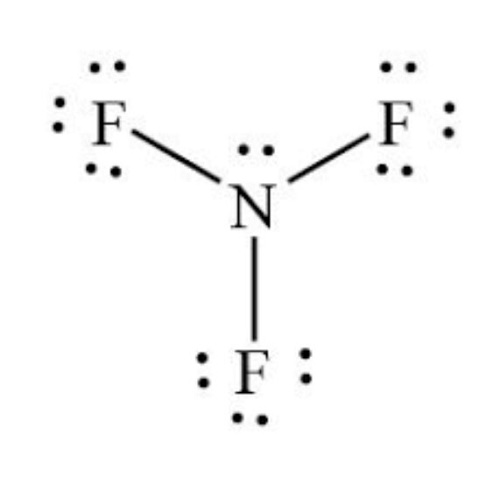
The core of nitrogen’s Lewis structure features five dots arranged around a central N symbol—two in a lone pair, three forming a trigonal planar set extending into single bonds with hydrogen, oxygen, or other atoms. The key resonance patterns highlight nitrogen’s capacity to achieve stable octets through covalent bonding, minimizing energy and maximizing stability.
Standard nitrogen exhibits a complete octet, fulfilling the eighth “rule” despite being a third-period element with only six electrons in the shell traditionally—solving a historical puzzle known as the “incomplete octet” conundrum.
The Octet Puzzle: Nitrogen’s Electron Strategy
In the 19th century, early chemists struggled to explain why nitrogen, despite having five valence electrons, needed fewer than eight in simple compounds. Nitrogen’s Lewis Dot Structure reveals a strategic compromise: it shares electrons—forming three bonds—rather than accepting or donating electrons adeptly. Unlike carbon, nitrogen rarely forms double or triple bonds in basic molecules, prioritizing three sigma bonds to complete its valence shell.
This selective bonding pattern reduces electron repulsion and maintains structural integrity.
For example, ammonia (NH₃) illustrates nitrogen’s bonding behavior: - A central nitrogen atom bonded to three hydrogen atoms, with one lone pair of electrons. - The three N–H sigma bonds and lone pair give nitrogen two bond domains and one lone domain, shaping its trigonal pyramidal geometry. - The structure’s electron distribution explains NH₃’s basicity—its lone pair readily accepts protons (H⁺)—a hallmark of nitrogen’s reactivity in aqueous solutions.
From Ammonia to Amides: Nitrogen’s Role in Key Molecules
Beyond ammonia, nitrogen’s Lewis structure underpins complex biomolecules and industrial chemicals.
In amino acids, the amine group (–NH₂) embodies nitrogen’s dual identity: a nucleophile with a lone pair and a site for protonation. Similarly, in carboxylic acids, nitrogen’s derivatives influence acidity and hydrogen bonding networks.
Consider nitriles (R–C≡N), where nitrogen forms three strong triple bonds. Its Lewis structure shows a carbon triple-bonded to nitrogen, with one lone pair and one additional electron: - This arrangement results in a highly electron-deficient nitrogen, making nitriles potent electrophiles.
- The triple bond’s rigidity creates extreme bond polarity and high bond energy, rendering nitriles stable yet reactive in nucleophilic additions. “The nitrogen in nitriles is a playground of reactivity,” explains Dr. Marcus Lin, specialized in organic synthesis.
“Its structure dictates not just stability, but reaction fate.”
Resonance and Molecular Stability in Nitrogen Chemistry
While structure diagrams show single bonds, resonance offers a deeper truth: nitrogen often participates in delocalized electron systems. In species like nitrate ion (NO₃⁻), nitrogen is central but constrained by resonance.
Though the Lewis structure for nitrate draws three equivalent bonds, actual bonding involves resonance—electron density shared across oxygen atoms.
This delocalization stabilizes the ion by spreading charge and lowering energy, a phenomenon invisible in isolated dot diagrams but critical for reaction mechanisms. “Visualizing resonance confirms nitrogen’s electron mobility,” says chemist Lin. “It explains why nitrogen-containing ions are so prevalent in catalysis and biological systems.”


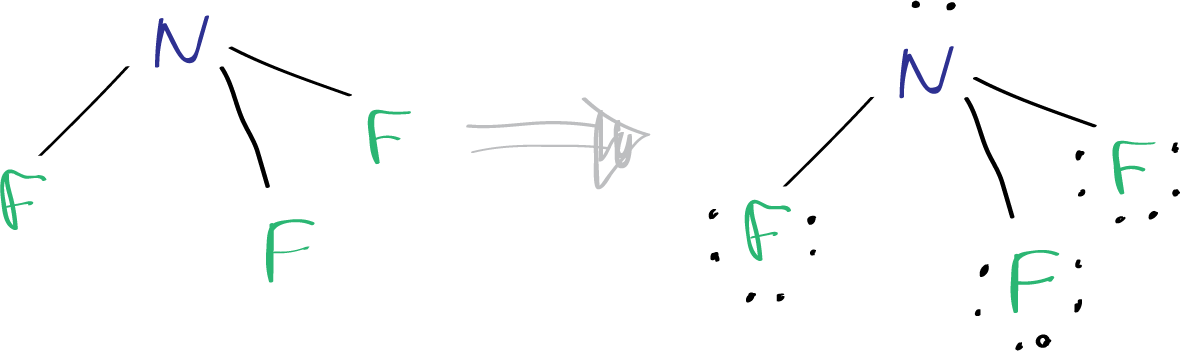
Related Post

Where Are They Now Original Chicago Band Members

Gem Swap 2: Rewrite Wealth with Strategic Gem Trading in a Dynamic Digital Arena
The Quiet Artistry of Nigel Slater Husband: Crafting Edible Bonds That Define a Culinary Legacy
A Tribute to Kelly Preston’s Cinematic Legacy: Crafting Class, Grace, and Complexity on Screen

