Claire Robinson Husband Unlocks the Hidden Risks of Gene Editing: Why CRISPR Is More Controversial Than We Think
Claire Robinson Husband Unlocks the Hidden Risks of Gene Editing: Why CRISPR Is More Controversial Than We Think
Public and scientific scrutiny of gene editing has intensified in recent years, with Claire Robinson Husband emerging as a leading voice exposing the darker undercurrents of CRISPR technology. Far from the utopian promise of eradicating disease, Robinson’s deep dive into the science, ethics, and real-world implications reveals a landscape fraught with unintended consequences, regulatory gaps, and profound moral dilemmas. Her investigative work underscores that while gene editing holds extraordinary potential, its application demands far greater caution than current policies suggest.
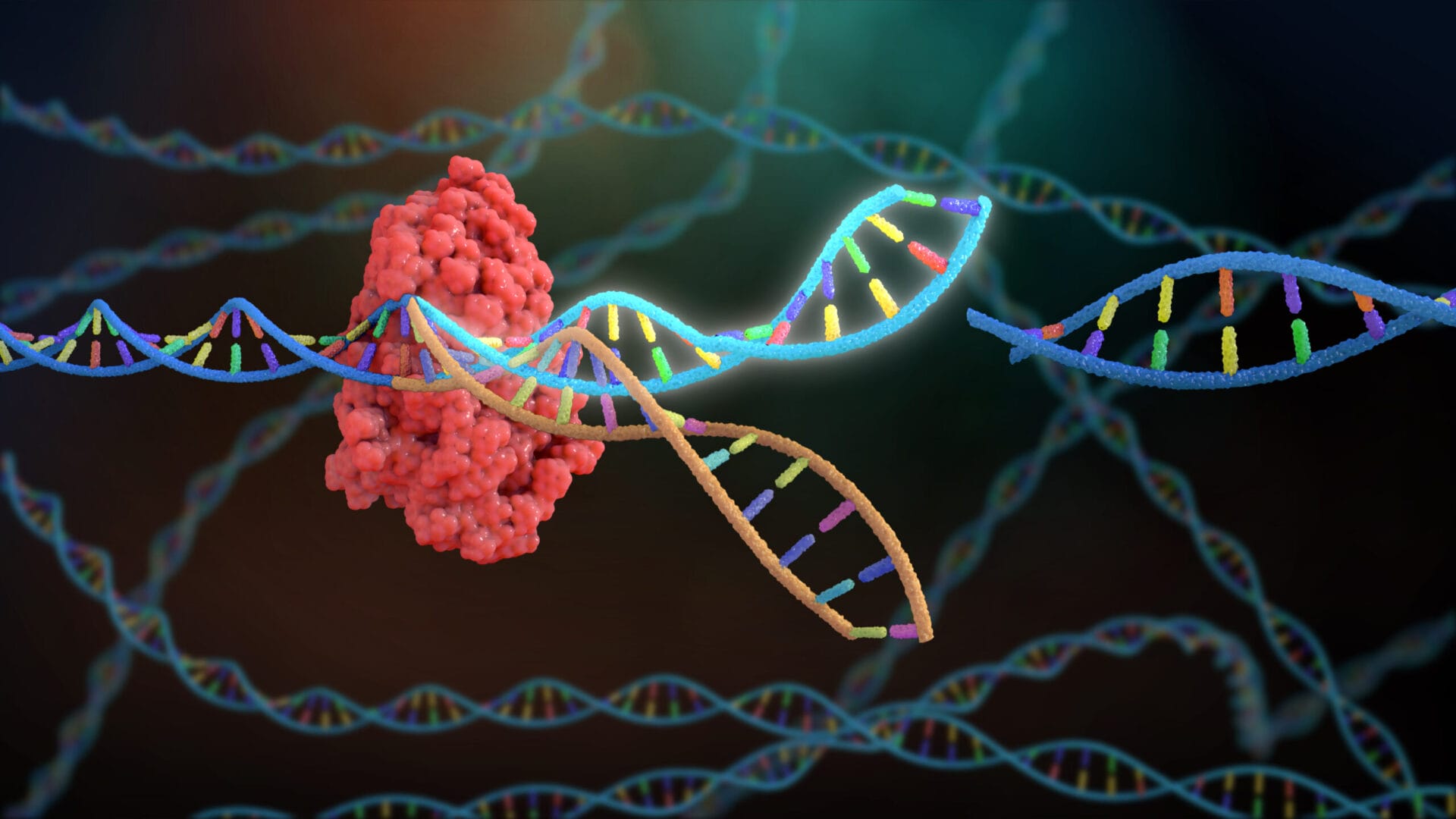
The Promise and Peril of Precision: CRISPR’s Scientific Foundations At the heart of modern genetic innovation stands CRISPR-Cas9—a molecular tool that functions like biological scissors, enabling scientists to edit DNA with unprecedented precision. First identified in bacteria as a defense system, CRISPR has revolutionized biomedical research, opening doors to treatments for genetic disorders such as sickle cell anemia and certain cancers. Yet, as Claire Robinson Husband emphasizes, technical precision does not eliminate risk.
Off-target mutations—where CRISPR alters unintended genetic sequences—remain a persistent challenge, with the potential to trigger unforeseen health complications or ecological disruptions. The technology’s power extends beyond humans. Agricultural applications of CRISPR promise drought-resistant crops and pest-resistant plants, but environmental impacts of gene-edited organisms remain poorly monitored.
Robinson highlights how edited genes can spread through wild populations, threatening biodiversity and creating “superweeds” immune to conventional control methods. This unpredictable ripple effect challenges the assumption that gene editing is inherently a safe, controlled science. From Laboratory to Liv: Real-World Applications and Unanswered Questions In recent years, CRISPR has moved from theoretical research to tangible clinical trials.
Famed cases like the use of gene editing to treat familial amyloidosis—a rare, fatal condition—have fueled optimism. Yet, these breakthroughs pose thorny ethical questions. Robinson meticulously cites incidents where early trials prioritized scientific success over patient safety, sparking heated debate within medical and bioethical circles.
Beyond medicine, the commercial drive behind editing crops, livestock, and even humans introduces additional layers of complexity. Companies race to bring gene-edited products to market, often ahead of comprehensive safety assessments. Robinson stresses that current regulatory frameworks, particularly in the U.S.
and EU, lag behind technological advances, allowing innovation to outpace oversight. She interviews researchers who note, “We’re editing life at a scale no one fully understands—yet we grant commercial approval without fully knowing what we’ve unleashed.” The Shadow of Heredity: Germline Editing and Its Global Implications Perhaps the most contentious frontier is germline editing—modifying genes in embryos so changes pass to future generations. Proponents argue it could eliminate hereditary diseases like Huntington’s or cystic fibrosis, but Robinson paints a cautionary picture.
The irreversible nature of germline edits means errors become permanent, echoing dark parallels with past genetic experimentation. The 2018 case of Chinese scientist He Jiankui—who created the first gene-edited babies—galvanized global outrage but failed to halt momentum. Robinson highlights ongoing efforts in nations with weaker regulatory enforcement, warning that unregulated “genetic tourism” threatens to turn human reproduction into a market-driven enterprise with deep ethical fissures.
Robinson recounts insights from international bioethicists: “We’re standing at a crossroads. Without global consensus and enforceable safeguards, CRISPR risks deepening inequality, eroding human dignity, and violating the principle of consent for future generations.” This absence of unified governance, she argues, is a gap thicker than the DNA strands scientists routinely slice. Societal Impact: Equity, Access, and the Risk of a Genetic Divide Beyond science and ethics, CRISPR raises urgent social questions.
Who benefits from gene editing? And who is at risk of being left behind? Robinson documents how high costs threaten to make genetic therapies accessible only to the wealthy, entrenching existing health disparities.
In wealthy nations, precision medicine advances rapidly, but in lower-income regions, basic healthcare remains elusive—despite CRISPR’s potential to combat tropical diseases or enhance food security. Equity concerns extend to agriculture, where CRISPR-edited crops could boost yields. Yet smallholder farmers may face corporate control over seeds, with patent rights limiting autonomy and deepening dependency.
Robinson warns, “Technological progress must not serve privilege—it must be guided by justice.” Arming Public Discourse: What You Need to Know Before the Gene Revolution Accelerates Claire Robinson Husband’s work calls for an urgent shift in public and policy awareness. While CRISPR offers transformative benefits, its risks demand rigorous, transparent oversight. Her advocacy centers on three key priorities: strengthening international regulatory frameworks, ensuring broad stakeholder engagement in decision-making, and investing in long-term ecological and health impact studies.
Public understanding remains fragmented. Surveys show growing public interest in gene editing, yet confusion persists over terminology and risks. Robinson champions science communication that empowers people—not just experts—to shape the future.
As she states, “The next chapter of human biology is being written. History will judge whether we wield this power wisely—or recklessly.” In balancing innovation with caution, the world stands at a pivotal moment. With every double helix cut, society must confront not just what CRISPR can do—but what it should.
Claire Robinson Husband’s commitment to truth and transparency serves as a vital compass in navigating this unprecedented frontier.
Claire Robinson Husband’s investigative lens brings clarity to a tangled era of genetic intervention. Her work reminds us that breakthroughs carry legacies—potters shaping clay must consider how artistry affects generations.
The path forward demands vigilance, equity, and humility. The stakes are nothing less than the integrity of life itself.


Related Post
Rebel Drops Seductive Reminder For Fans With Cheeky Underwear Photo

Carmelo Anthony’s Daughter Who Is the Mother: The Quiet Strength in His Legacy

römàn 1–20: Decoding the 20 Core Pillars Shaping Modern Innovation
Forensic Documentation: Analyzing the Circumstances of the Dayvon Bennett Affair Outcomes

