Breaking Down Hydrolyse ATP: The Catalyst Behind Energy Transformation in Cells
Breaking Down Hydrolyse ATP: The Catalyst Behind Energy Transformation in Cells
At the core of cellular energy metabolism lies a molecular marvel: the hydrolysis of adenosine triphosphate (ATP), a process so fundamental that it drives nearly every biological function—from nerve impulses to muscle contractions and biosynthetic pathways. Known primarily as the “energy currency” of life, ATP functions not merely as a storage molecule but as an active participant in energy transfer, particularly through hydrolysis. The enzyme-driven hydrolysis of ATP cleaves high-energy phosphate bonds, releasing energy stored in its terminal phosphoanhydride linkage, and this transformation powers cellular machinery with remarkable efficiency.
Understanding how ATP hydrolysis works—both chemically and biologically—illuminates the elegant mechanics underlying life’s energy economy.
The Chemistry of ATP Hydrolysis: A Molecular Mechanism
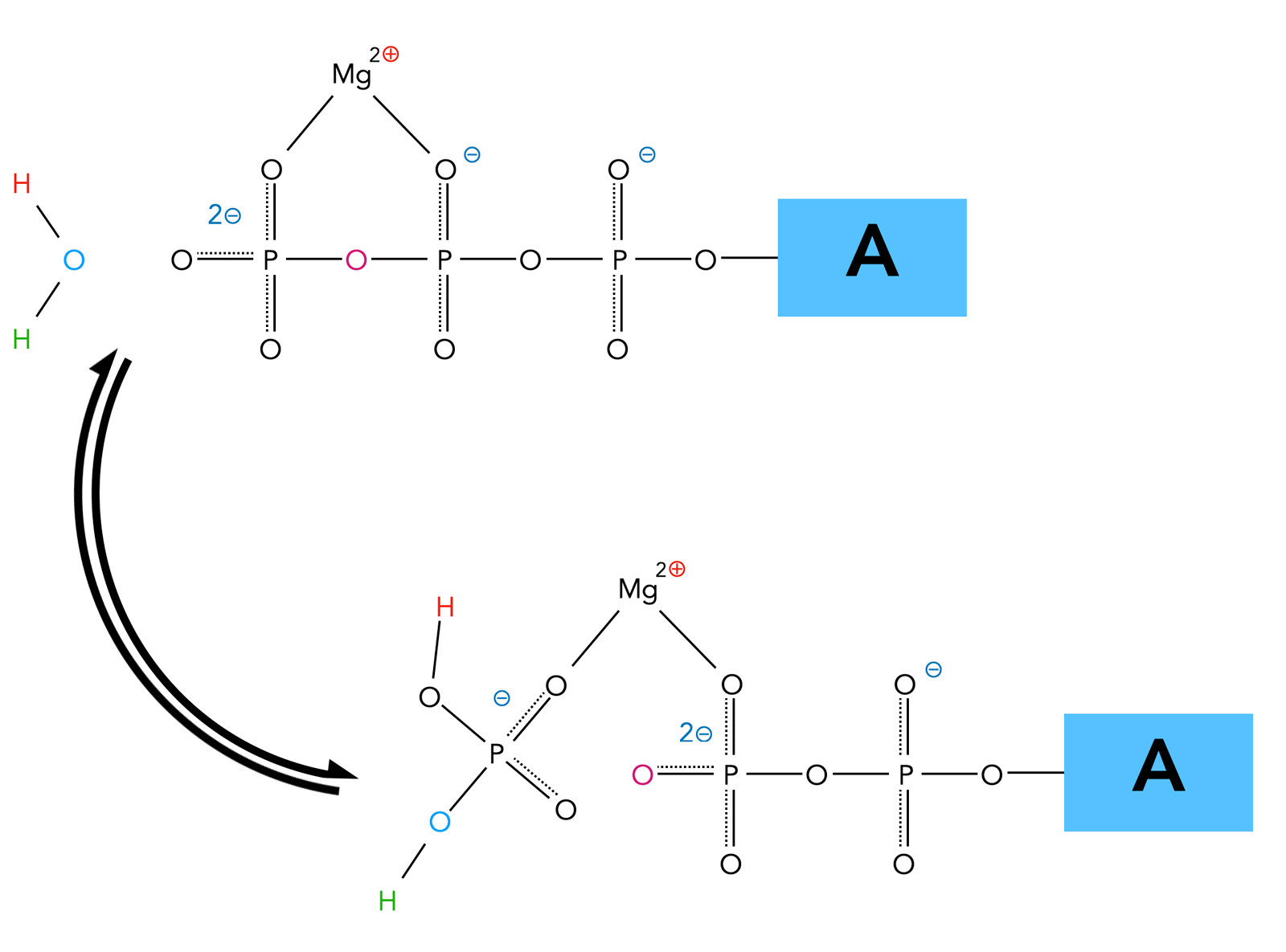
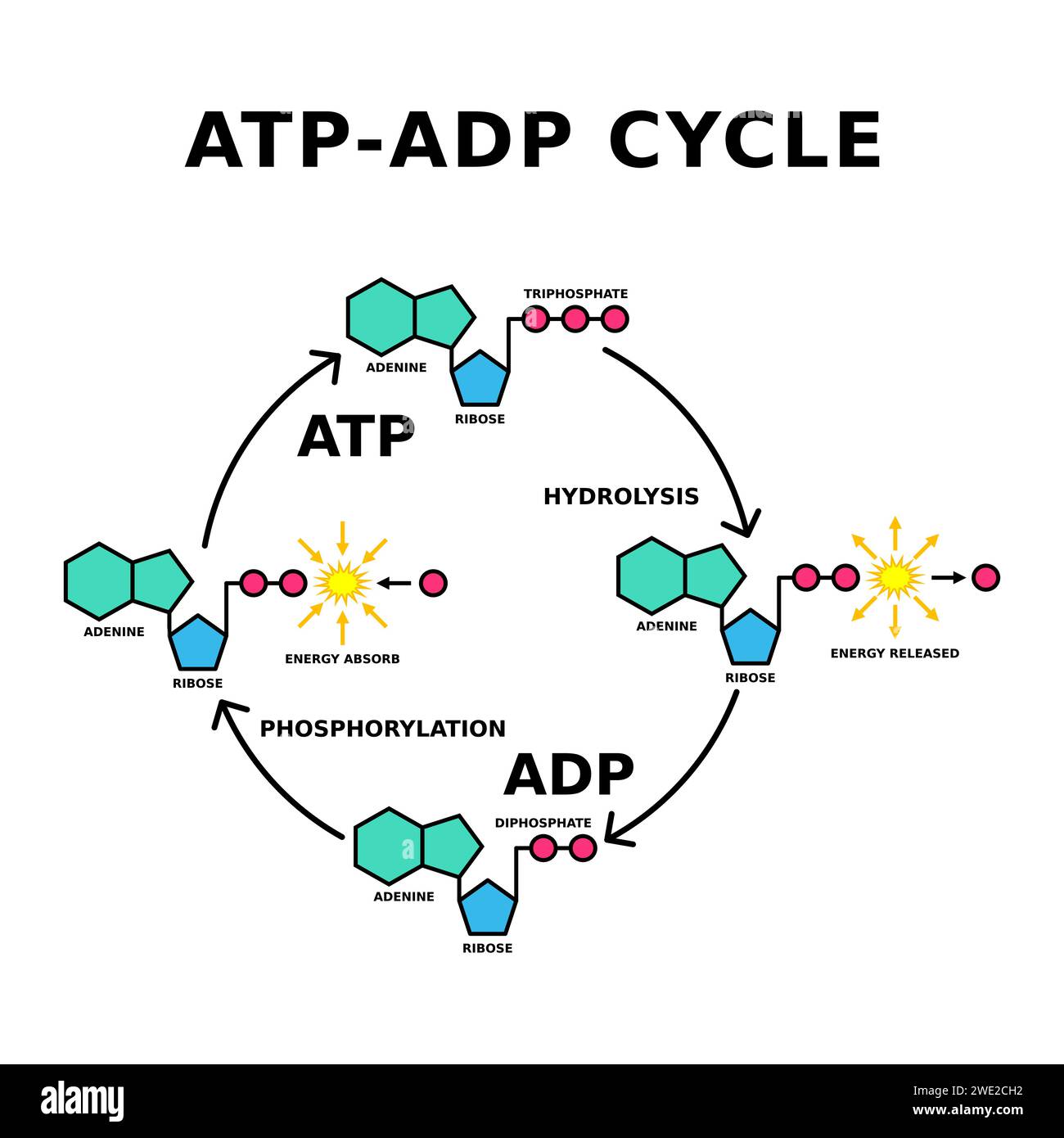
ATP (adenosine triphosphate) consists of three primary components: an adenine base, a ribose sugar, and three phosphate groups linked by rapidly breakable phosphoanhydride bonds. Hydrolysis occurs when a water molecule splits the bond between the second and third phosphate, converting ATP into adenosine diphosphate (ADP) and inorganic phosphate (Pi). This reaction releases approximately 30.5 kilojoules per mole under standard conditions—enough energy to drive numerous biochemical processes.
The reaction is exergonic, meaning it proceeds spontaneously due to favorable changes in Gibbs free energy (ΔG°’ ≈ -30.5 kJ/mol). The instability of the terminal phosphate bond arises from electrostatic repulsion and resonance stabilization in the being-via transition state, making hydrolysis energetically favorable.
pH and ionic conditions profoundly affect hydrolysis rates, though ATP remains stable within physiological pH (7.2–7.4). Enzymes such as ATPases accelerate hydrolysis by ~10^17 fold, enabling biological systems to harness free energy efficiently without wasteful spontaneous breakdown.
This enzymatic control ensures ATP is used precisely when and where needed.
Enzymes that Harness Hydrolysis: ATPases and Beyond
At the heart of ATP hydrolysis are a diverse family of enzymes collectively called ATPases. These proteins bind ATP, induce conformational changes, and facilitate bond cleavage by stabilizing the transition state.
Key classes include:
- Secreted ATPases (e.g., ATP synthase): Found in mitochondria and chloroplasts, ATP synthase functions as a molecular turbine, coupling proton motive force to ATP formation during oxidative phosphorylation and photophosphorylation. In reverse, intact enzyme complexes hydrolyze ATP to pump protons across membranes—a dual role central to energy transduction.
- Cytosolic ATPases (e.g., myosin, Na⁺/K⁺-ATPase): In muscle cells, myosin hydrolyzes ATP to generate mechanical energy during contraction, using released Pi to power cyclic interaction with actin. Ion pumps like Na⁺/K⁺-ATPase maintain cellular electrolyte gradients critical for nerve signaling and osmotic balance.
- Hydrolases in Catabolic Pathways: Enzymes such as ATP-dependent kinases and phosphatases regulate metabolic networks by adding or removing phosphate groups, modulating enzyme activity and signaling cascades.
These specialized enzymes transform hydrolysis from a mere chemical reaction into a regulated, multidirectional energy-switch.
Their precision ensures metabolic flux matches cellular demand, acting as both fuel and executor of biochemical transformation.
Energy Output and Cellular Efficiency: The Power Behind the Pulse
While the free energy yield from ATP hydrolysis is substantial, biological systems extract maximal value through spatial and temporal compartmentalization. The energy released is not dissipated—it is scavenged by immediate downstream processes. For example, in muscle contraction: - The energy from ATP hydrolysis by myosin drives filament sliding.
- This mechanical work is efficiently converted into kinetic energy via actin binding. - Nicht-productive energy—such as heat—is minimized through enzyme alignment and proximity control.
Similarly, ATP synthase generates ATP with near-quantum efficiency, converting electrochemical gradients into chemical bonds with minimal entropy loss.
Under optimal conditions, up to 90–95% of the energy from proton flow is conserved, approached only by synthetic energy storage systems. This efficiency positions ATP hydrolysis as nature’s gold standard for energy currency.
Beyond contractile tissues, ATP hydrolysis powers:
- Nerve impulse transmission via Na⁺/K⁺-ATPase maintaining resting membrane potentials
- Protein synthesis driven by aminoacyl-tRNA synthetases using ATP to charge tRNAs
- DNA replication and repair enzymes relying on ATP for strand separation and nucleotide addition
The ubiquity and precision of hydrolysis underscore ATP’s irreplaceable role—its cyclical breakdown and renewal defines cellular vitality.
Hydrolysis and Regulation: Balancing Energy Use and Conservation
Cellular energy economy hinges on tightly regulated hydrolysis. Uncontrolled ATP breakdown would drain reserves rapidly, while insufficient use compromises function.
This balance is maintained through allosteric enzymes and feedback mechanisms. For instance, phosphofructokinase—key to glycolysis—is inhibited by high ATP levels, preventing wasteful turnover when energy is abundant. Conversely, AMP-activated protein kinase (AMPK) activates pathways promoting ATP generation when energy levels fall, triggering catabolic processes to restore balance.
Moreover, many ATP-dependent processes couple hydrolysis to downstream reactions: - ATPase activity powers vesicle trafficking by endogenous proton pumps.
- ATP-driven remodeling enzymes adjust chromatin structure, enabling gene expression in response to metabolic signals.
This integration ensures ATP hydrolysis fuels not isolated events but interconnected networks sustaining life.
Clinical and Technological Frontiers: Exploiting Hydrolysis Mechanisms
Understanding ATP hydrolysis has far-reaching implications. In medicine, targeting ATPases offers strategies against diseases rooted in energy failure—such as neurodegenerative disorders, where mitochondrial ATP synthesis falters, or ion pump deficiencies causing cystic fibrosis.
Drug development now focuses on modulating ATPase activity to restore cellular homeostasis.
Technologically, ATP hydrolysis inspires biohybrid systems: artificial motors mimic ATP synthase to power nanomachines, while enzymatic fuel cells convert biochemical ATP into electrical energy. These innovations highlight ATP’s dual identity—as nature’s energy unit and a blueprint for sustainable energy solutions.
The manipulation of hydrolysis pathways also advances synthetic biology, where engineered cells produce custom energy currencies or biosensors responsive to ATP concentration, enabling real-time metabolic monitoring.
From the rhythmic pulse of a muscle fiber to the quiet spark of a metabolic enzyme, ATP hydrolysis remains the silent engine of life. Its mechanism—simple in bond-breaking, complex in biological control—epitomizes nature’s elegance: harnessing exergonic reactions not for chaos, but for purposeful, regulated energy transfer.
As science deepens its insight into this process, so too does our capacity to harness energy, heal, and innovate.


Related Post

Debra Bollman’s Legacy: A Blueprint for Excellence in Investigative Journalism
Revealed: The Truth About the 'Adam Pearson Wife' Search
Charlie Lenehan Songs Bio Wiki Age Girlfriend Baby and Net Worth

Ratio Americano: The Bold Brew Revolutionizing American Coffee Culture

