Atom Unlocked: Decoding Sodium’s Structure at the Quantum Level
Atom Unlocked: Decoding Sodium’s Structure at the Quantum Level
Beneath the simplicity of everyday materials lies a world of intricate atomic architecture—and nowhere is this more evident than in the sodium atom. As one of the most fundamental alkali metals, sodium offers a gateway into the behavior of electrons, energy states, and chemical reactivity, all governed by the precise arrangement of its nucleus and electron cloud. By examining the model of a sodium atom, scientists and students alike uncover how its unique electron configuration drives its vivid yellow flame, remarkable conductivity, and explosive reactivity.
This article explores sodium’s atomic model in detail, revealing how its structure shapes not just the element itself, but much of modern materials science and industrial chemistry.
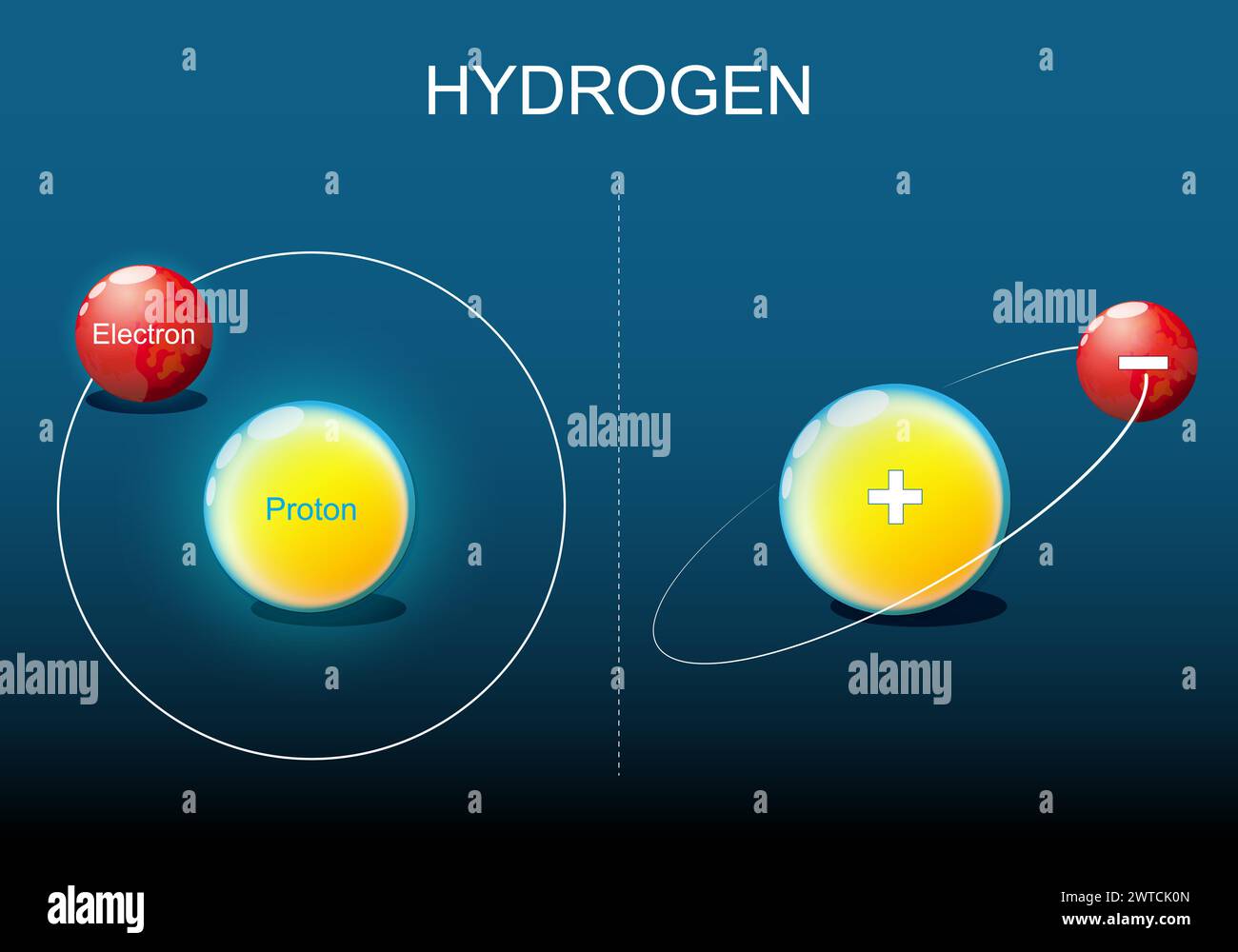
At the core of sodium’s identity is a nucleus composed of 11 protons and 11 neutrons, anchoring the atom’s identity. Orbiting this core are three electron shells, with the outermost—known as the valence shell—containing a single electron.
This lone valence electron defines sodium’s chemical behavior: it is loosely bound, making sodium highly reactive, particularly with water. “Sodium’s single outer electron is nature’s invitation for interaction,” notes Dr. Elena Torres, a quantum chemist at Argonne National Laboratory.
“This electron is missing a strong anchoring force, and that’s precisely why sodium dances with halogens, fluorine included, forming compounds that power everything from fertilizers to battery technologies.”
Visualizing sodium’s electron configuration reveals a systematic filling of subshells following the Aufbau principle. The 1s orbital holds two electrons, followed by 2s², and then 2p⁶, elevating to 3s¹—the lone electron responsible for sodium’s metallic and electrochemical traits. “What’s striking,” explains Dr.
Rajiv Mehta, a physicist specializing in atomic structure, “is how predictable this filling is. Unlike transition metals, sodium lacks d-electrons, which simplifies its electronic transitions and correlates directly with its characteristic emission spectrum—most notably the bright yellow glow seen when sodium vapor ignites.”
Examining sodium’s electron energy levels, the 3s orbital is the only occupied state outside the core. “Electrons here live in a delicate balance—enough energy to leave easily, but not so much that escape occurs spontaneously under normal conditions,” Mehta explains.
In gas-phase models, sodium’s electron density appears concentrated in a single orbital, spreading predictably across space. This spatial distribution influences how sodium atoms interact with light and other atoms, contributing to its strong absorption in the visible spectrum and defining its utility in lasers and spectroscopy.
When modeling sodium as an atom, the nuclear charge and electron shielding become critical factors.
With 11 protons, the positive charge pulls valence electrons strongly, yet the absence of electron shielding effects—common in more complex atoms—means sodium’s outermost electron experiences nearly full nuclear attraction. Quantum mechanical calculations confirm that this electron occupies a 3s state with an average energy of approximately –3.1 eV, a value that governs its ionization energy: just 501 kJ/mol. This low ionization barrier explains sodium’s ready donation of electrons, a behavior exploited in electrochemical cells and metallurgical processes.
Another defining feature of sodium’s atomic model is its cascade of possible electron transitions. Once excited—say, by thermal energy or photon absorption—the sodium atom’s electron can jump to higher orbitals, though such transitions require precise energy quanta. The most prominent transition, responsible for its yellow emission at ~589 nm, occurs when the 3s electron drops to the 3p level.
“This energy difference corresponds to a photon of yellow light, making sodium one of the key elements in flame tests and industrial flame detectors,” Mehta notes. In solid alloys, these transitions modulate conductivity and optical responses, underpinning sodium’s role in industrial applications.
Visual representations of sodium atoms often depict the nucleus—dense and centralized—surrounded by concentric electron shells.
In three-dimensional models, this structure accentuates sodium’s metallic nature: the valence electron is delocalized, free to move across a lattice of positive ions. This electron mobility, explained by bande theory, is why sodium conducts electricity when molten or dissolved in water. The model thus captures not just atomic geometry but the dynamic behavior underlying sodium’s utility in batteries, heat transfer, and chemical synthesis.
While the sodium atom is often introduced as a simple exAMPLE in chemistry classrooms, its model reveals layers of physical and chemical complexity. From electron shell stability to transition energies, each aspect reflects fundamental principles of quantum mechanics and periodic trends. “Studying sodium,” says Dr.
Torres, “is like holding a lens to the behavior of alkali metals as a whole. Its simplicity belies profound implications for materials science, energy storage, and even astrophysics—where sodium lines are signatures in stellar spectra.”
The model of a sodium atom, though grounded in model chemistry rather than atomic reality (as income atoms are approximations), captures the essence of electronic structure that governs sodium’s behavior. By understanding how protons and electrons interact at the quantum level, researchers and engineers harness sodium’s properties—whether in sodium vapor lamps illuminating streets, sodium-ion batteries emerging as smart energy storage, or industrial rods reacting dramatically with water.
The atomic architecture of sodium is not just a scientific curiosity; it is a blueprint for innovation.
In essence, the sodium atom stands as a stand-in for reactive metals at the heart of modern technology. Its electron dynamics, energy levels, and nuclear configuration converge to explain a spectrum of observable phenomena—from the crackle of a flame to the flow of electricity in circuits.
As scientific models grow ever more precise, the humble sodium atom continues to illuminate the invisible forces that shape our world.



Related Post
Discover The Shocking Truth: Unveiling The Killer Behind The 'Who Killed Chris Chahal In' Mystery

Transform Your Mobile Experience: Zedge App Wallpapers, Ringtones & Unique Audio Cuts Power Your Digital Style
What is John Millers Net Worth in 2023

Unlock Immersive Gaming with the Revolutionary Platform of www.Bloxa.Us

