Acetic Acid Is a Weak Acid Because of Its Limited Dissociation—But What That Means Matters
Acetic Acid Is a Weak Acid Because of Its Limited Dissociation—But What That Means Matters
Acetic acid, the foundational molecule behind vinegar and essential in biological and chemical systems, is classified not as a strong acid but a weak acid—a distinction rooted in its incomplete ionization in aqueous solution. This subtle classification has far-reaching implications for its behavior in chemistry, biology, and industry. Understanding why acetic acid acts as a weak acid reveals the nuanced nature of acid strength and its practical significance across fields ranging from food science to environmental chemistry.
What Defines a Weak Acid? A weak acid is one that does not fully release hydrogen ions (H⁺) when dissolved in water. According to the definition established by acid-base chemistry, such substances establish an equilibrium between dissociated ions and undissociated molecules in solution. This equilibrium favors the original molecule far more than in strong acids, reflected in lower acid dissociation constants (Ka).
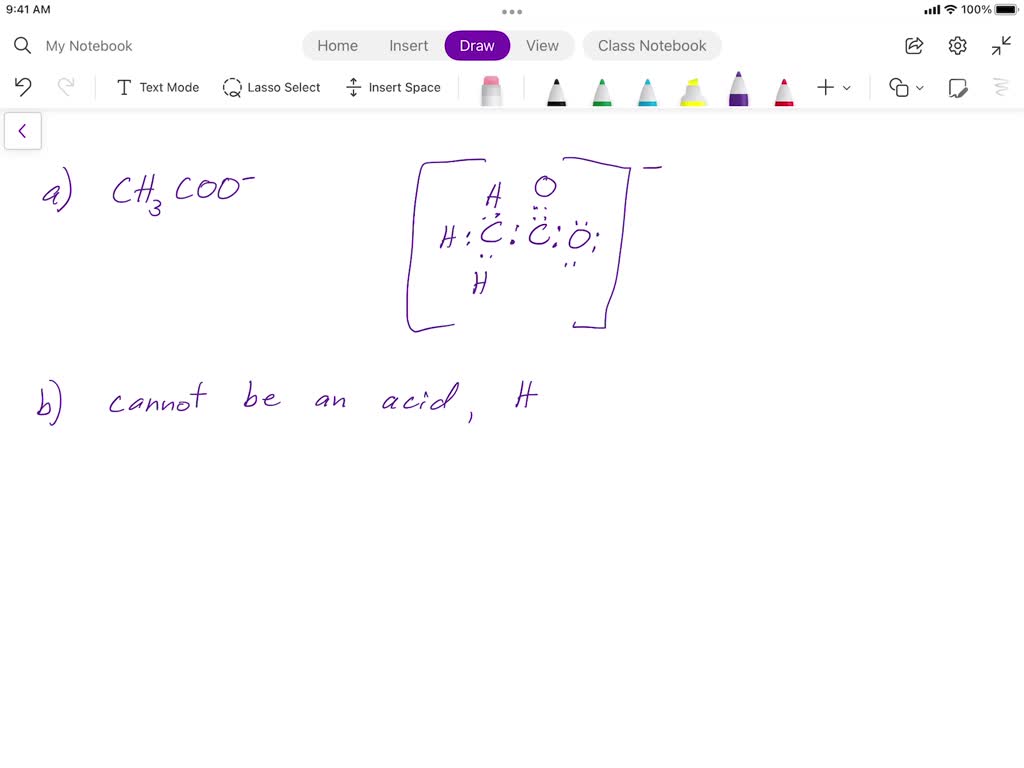
For acetic acid, this means only a small fraction of molecules break apart—explaining its muted ability to donate protons compared to mineral acids like hydrochloric or sulfuric acid. Molecular Behavior: Why Acetic Acid Weakens Slowly Acetic acid’s weakness stems from the structural stability of its conjugate base, the acetate ion (CH₃COO⁻). The resonance stabilization within acetate—where negative charge delocalizes between two oxygen atoms—delays complete deprotonation.
This molecular design creates a persistent tug-of-war between undissociated acetic acid and its ions, resulting in a low Ka value of approximately 1.8 × 10⁻⁵. In practical terms, only about 1.34% of acetic acid molecules dissociate in water, a fraction small enough to keep pH levels moderate and reactivity gentle. This restrained dissociation defines its weak acid identity.
Unlike strong acids, which fully shed H⁺ ions, acetic acid resists full ionization, producing a solution with a pH typically between 2.8 and 3.0 at 5% concentration. The balance between proton-sharing and retention shapes its cautious role in acid-base reactions, making it predictable yet mild in chemical environments. Quantifying Weakness: The Role of Dissociation Equilibrium At the heart of acetic acid’s weak nature is the reversible dissociation reaction: CH₃COOH ⇌ CH₃COO⁻ + H⁺ Equilibrium constants quantify this process.
The acidic dissociation constant, Ka, reflects the extent to which the acid donates a proton. For acetic acid, Ka ≈ 1.8 × 10⁻⁵ means equilibrium lies heavily skewed toward the left side, favoring the neutral molecule. This partial ionization means not every drop of vinegar contributes maximum acidity—its effectiveness depends on concentration and context.
In concentration-dependent applications—such as antiseptic solutions or biochemical pathways—concentration moderates acetic acid’s mildness. Doubling vinegar’s strength doubles available H⁺, but not to the explosive level seen with HCl. This controlled release supports its widespread use in natural and industrial processes without overwhelming sensitive systems.
Real-World Implications of Acetic Acid’s Weakness Acetic acid’s weak acidity is not a limitation but a strategic advantage. In biological systems, enzymes rely on precise pH control; weak acids like acetic acid help buffer cellular environments, preventing dangerous spikes in acidity. In food preservation, its modest acidity inhibits pathogenic microbes while allowing flavors to develop gently over time—though robust acids are needed for sterilization.
Industrially, acetic acid’s controlled behavior enables safe, scalable applications. From textile dyeing, where gradual acidification prevents fiber damage, to pharmaceutical formulations requiring steady pH buffering, its weak nature ensures reliability. Even in environmental remediation, acetic acid aids in mild metal extraction without destabilizing surrounding ecosystems.
The Broader Significance of Weak Acid Behavior Understanding why acetic acid is a weak acid goes beyond nomenclature—it illuminates how molecular structure dictates function. This principle underpins much of organic and inorganic chemistry: strong acids thrive in complete ionization, but weak acids like acetic acid play nuanced, reliable roles where mild acidity is preferred. Their behavior shapes everything from household cleaning products to metabolic pathways, proving that weakness in chemistry can be deliberate and vital.
The story of acetic acid is one of equilibrium and control—a molecule that, though reluctant to share protons, fulfills its chemical purpose with precision. Recognizing its weak acid identity demystifies its role, revealing why it remains indispensable in both science and daily life. In essence, acetic acid’s status as a weak acid stems from its structural stability and balanced dissociation kinetics.
This subtle characteristic, far from a flaw, defines its utility—offering strength through subtlety, and demonstrating how even the kindness of a weak acid can have profound impact.



Related Post
Lil Baby Height How tall is Lil Baby Background Career Net Worth Girlfriend
IDJ Carlos Augusto: Unraveling the Mystery Behind DJ Bloodline's Meteoric Rise and Enduring Legacy

Who Shot 50 Cent: The Bombing That Shook Hip-Hop and Ignited a Defining Myth
Erome Size 7 Sparks Intense Reaction Across Online Users, Igniting Viral Debate and Unprecedented Engagement

